Abstract
Purpose
Liver regeneration after partial hepatectomy (PH) occurs in conditions of reduced oxygen supply. HIF prolyl hydroxylase enzymes (PHD1, PHD2, and PHD3) are oxygen sensors involved in adaptive response to hypoxia. Specific functions of these PHD enzymes in liver regeneration have, however, remained enigmatic. Here, we investigated the significance of PHD1 in liver regeneration following hepatectomy.
Methods
Liver regeneration was studied in PHD1-deficient (PHD1−/−) and wild type (WT) mice subjected to 80 % hepatectomy. For in vitro analyses, hepatocytes were isolated from PHD1−/− and WT livers. Cell cycle progression was studied via FACS-based analysis of nuclear DNA profile. Transcription factor binding assays, qRT-PCR, and immunoblotting were applied to study the relevance of PHD1 downstream effectors during liver regeneration.
Results
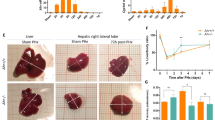
Liver regeneration was significantly enhanced in PHD1−/− mice compared to WT littermates. This effect was due to enhanced proliferation rather than to hypertrophy of liver cells. Cell cycle progression was significantly enhanced, and transcriptional activity of the cell cycle regulator c-Myc was increased in PHD1-deficient hepatocytes. These changes coincided with increased expression of cyclin D2, a cell cycle-promoting c-Myc target, and decreased expression of the cell cycle-delaying c-Myc target p21.
Conclusions
Loss of PHD1 enhances liver regeneration by boosting hepatocyte proliferation in a c-Myc-dependent fashion. PHD1 might, therefore, represent a potential target to facilitate liver regeneration after surgical resection.





Similar content being viewed by others
References
Poon RT, Fan ST, Lo CM et al (2004) Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg 240(4):698–708, discussion 708-610
Reissfelder C, Rahbari NN, Koch M et al (2011) Postoperative course and clinical significance of biochemical blood tests following hepatic resection. Br J Surg 98(6):836–844
Rahbari NN, Reissfelder C, Koch M, Elbers H, Striebel F, Buchler MW, Weitz J (2011) The predictive value of postoperative clinical risk scores for outcome after hepatic resection: a validation analysis in 807 patients. Ann Surg Oncol 18(13):3640–3649
Kubota K, Makuuchi M, Kusaka K et al (1997) Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology 26(5):1176–1181
Hammond JS, Guha IN, Beckingham IJ, Lobo DN (2011) Prediction, prevention and management of postresection liver failure. Br J Surg 98(9):1188–1200
Michalopoulos GK (2007) Liver regeneration. J Cell Physiol 213(2):286–300
Abshagen K, Eipel C, Vollmar B (2012) A critical appraisal of the hemodynamic signal driving liver regeneration. Langenbecks Arch Surg 397(4):579–590
Kaelin WG Jr, Ratcliffe PJ (2008) Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell 30(4):393–402
Semenza GL (2009) Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiol (Bethesda) 24:97–106
Maeno H, Ono T, Dhar DK, Sato T, Yamanoi A, Nagasue N (2005) Expression of hypoxia inducible factor-1alpha during liver regeneration induced by partial hepatectomy in rats. Liver Int 25(5):1002–1009
Schmeding M, Boas-Knoop S, Lippert S et al (2008) Erythropoietin promotes hepatic regeneration after extended liver resection in rats. J Gastroenterol Hepatol 23(7 Pt 1):1125–1131
Rankin EB, Rha J, Selak MA, Unger TL, Keith B, Liu Q, Haase VH (2009) Hypoxia-inducible factor 2 regulates hepatic lipid metabolism. Mol Cell Biol 29(16):4527–4538
Tajima T, Goda N, Fujiki N et al (2009) HIF-1alpha is necessary to support gluconeogenesis during liver regeneration. Biochem Biophys Res Commun 387(4):789–794
Nishiyama Y, Goda N, Kanai M et al (2012) HIF-1alpha induction suppresses excessive lipid accumulation in alcoholic fatty liver in mice. J Hepatol 56(2):441–447
Kim WY, Safran M, Buckley MR et al (2006) Failure to prolyl hydroxylate hypoxia-inducible factor alpha phenocopies VHL inactivation in vivo. EMBO J 25(19):4650–4662
Yoon D, Okhotin DV, Kim B et al (2010) Increased size of solid organs in patients with Chuvash polycythemia and in mice with altered expression of HIF-1alpha and HIF-2alpha. J Mol Med (Berl) 88(5):523–530
Minamishima YA, Kaelin WG Jr (2010) Reactivation of hepatic EPO synthesis in mice after PHD loss. Science 329(5990):407
Fraisl P, Aragones J, Carmeliet P (2009) Inhibition of oxygen sensors as a therapeutic strategy for ischaemic and inflammatory disease. Nat Rev Drug Discov 8(2):139–152
Kiss J, Kirchberg J, Schneider M (2012) Molecular oxygen sensing: implications for visceral surgery. Langenbecks Arch Surg 397(4):603–610
Schneider M, Van Geyte K, Fraisl P et al (2010) Loss or silencing of the PHD1 prolyl hydroxylase protects livers of mice against ischemia/reperfusion injury. Gastroenterology 138(3):1143–1154, e1141-1142
Zhong Z, Ramshesh VK, Rehman H et al (2008) Activation of the oxygen-sensing signal cascade prevents mitochondrial injury after mouse liver ischemia-reperfusion. Am J Physiol Gastrointest Liver Physiol 295(4):G823–832
Bishop T, Gallagher D, Pascual A et al (2008) Abnormal sympathoadrenal development and systemic hypotension in PHD3−/− mice. Mol Cell Biol 28(10):3386–3400
Mazzone M, Dettori D, Leite de Oliveira R et al (2009) Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell 136(5):839–851
Greene AK, Puder M (2003) Partial hepatectomy in the mouse: technique and perioperative management. J Investig Surg 16(2):99–102
Dirkx R, Meyhi E, Asselberghs S, Reddy J, Baes M, Van Veldhoven PP (2007) Beta-oxidation in hepatocyte cultures from mice with peroxisomal gene knockouts. Biochem Biophys Res Commun 357(3):718–723
Mancone C, Conti B, Amicone L et al (2010) Proteomic analysis reveals a major role for contact inhibition in the terminal differentiation of hepatocytes. J Hepatol 52(2):234–243
Walmsley SR, Chilvers ER, Thompson AA et al (2011) Prolyl hydroxylase 3 (PHD3) is essential for hypoxic regulation of neutrophilic inflammation in humans and mice. J Clin Investig 121(3):1053–1063
Kiss J, Mollenhauer M, Walmsley SR et al (2012) Loss of the oxygen sensor PHD3 enhances the innate immune response to abdominal sepsis. J Immunol 189(4):1955–1965
Takeda Y, Costa S, Delamarre E et al (2011) Macrophage skewing by Phd2 haplodeficiency prevents ischaemia by inducing arteriogenesis. Nature 479(7371):122–126
Malato Y, Sander LE, Liedtke C, Al-Masaoudi M, Tacke F, Trautwein C, Beraza N (2008) Hepatocyte-specific inhibitor-of-kappaB-kinase deletion triggers the innate immune response and promotes earlier cell proliferation during liver regeneration. Hepatology 47(6):2036–2050
Wu JC, Merlino G, Fausto N (1994) Establishment and characterization of differentiated, nontransformed hepatocyte cell lines derived from mice transgenic for transforming growth factor alpha. Proc Natl Acad Sci USA 91(2):674–678
Gordan JD, Bertout JA, Hu CJ, Diehl JA, Simon MC (2007) HIF-2alpha promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell 11(4):335–347
Kountouras J, Boura P, Lygidakis NJ (2001) Liver regeneration after hepatectomy. Hepatogastroenterology 48(38):556–562
Harun N, Nikfarjam M, Muralidharan V, Christophi C (2007) Liver regeneration stimulates tumor metastases. J Surg Res 138(2):284–290
Christophi C, Harun N, Fifis T (2008) Liver regeneration and tumor stimulation—a review of cytokine and angiogenic factors. J Gastrointest Surg 12(5):966–980
Gordan JD, Thompson CB, Simon MC (2007) HIF and c-Myc: sibling rivals for control of cancer cell metabolism and proliferation. Cancer Cell 12(2):108–113
Baena E, Gandarillas A, Vallespinos M et al (2005) c-Myc regulates cell size and ploidy but is not essential for postnatal proliferation in liver. Proc Natl Acad Sci USA 102(20):7286–7291
Hurlin PJ, Huang J (2006) The MAX-interacting transcription factor network. Semin Cancer Biol 16(4):265–274
Huang LE (2008) Carrot and stick: HIF-alpha engages c-Myc in hypoxic adaptation. Cell Death Differ 15(4):672–677
Fernandez PC, Frank SR, Wang L et al (2003) Genomic targets of the human c-Myc protein. Genes Dev 17(9):1115–1129
Koshiji M, Kageyama Y, Pete EA, Horikawa I, Barrett JC, Huang LE (2004) HIF-1alpha induces cell cycle arrest by functionally counteracting Myc. EMBO J 23(9):1949–1956
Koshiji M, To KK, Hammer S, Kumamoto K, Harris AL, Modrich P, Huang LE (2005) HIF-1alpha induces genetic instability by transcriptionally downregulating MutSalpha expression. Mol Cell 17(6):793–803
Khan Z, Michalopoulos GK, Stolz DB (2006) Peroxisomal localization of hypoxia-inducible factors and hypoxia-inducible factor regulatory hydroxylases in primary rat hepatocytes exposed to hypoxia–reoxygenation. Am J Pathol 169(4):1251–1269
Rankin EB, Biju MP, Liu QD et al (2007) Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J Clin Invest 117(4):1068–1077
Rankin EB, Rha J, Unger TL et al (2008) Hypoxia-inducible factor-2 regulates vascular tumorigenesis in mice. Oncogene 27(40):5354–5358
Klemm K, Eipel C, Cantre D, Abshagen K, Menger MD, Vollmar B (2008) Multiple doses of erythropoietin impair liver regeneration by increasing TNF-alpha, the Bax to Bcl-xL ratio and apoptotic cell death. PLoS One 3(12):e3924
Aragones J, Schneider M, Van Geyte K et al (2008) Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nat Genet 40(2):170–180
Goralczyk AD, Obed A, Beham A, Tsui TY, Lorf T (2011) Posterior cavoplasty: a new approach to avoid venous outflow obstruction and symptoms for small-for-size syndrome in right lobe living donor liver transplantation. Langenbecks Arch Surg 396(3):389–395
Acknowledgments
This study was supported by the Emmy Noether Program of the Deutsche Forschungsgemeinschaft (DFG; Grant 947/2-1 to M.S.) and in the framework of the Clinical Research Group KFO 227 by the DFG (Grant 947/4-1 to M.S.).
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Martin Mollenhauer and Judit Kiss contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 135 kb)
Rights and permissions
About this article
Cite this article
Mollenhauer, M., Kiss, J., Dudda, J. et al. Deficiency of the oxygen sensor PHD1 augments liver regeneration after partial hepatectomy. Langenbecks Arch Surg 397, 1313–1322 (2012). https://doi.org/10.1007/s00423-012-0998-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-012-0998-5