Abstract
Introduction
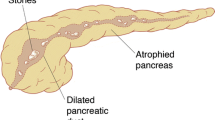
Chronic pancreatitis (CP) is a disease with enormous social and personal impact. It is most commonly caused by the abuse of alcohol combined with nicotine. CP is usually characterised by an inflammatory mass located in the pancreatic head. Its natural course is characterised by persistent or recurrent painful attacks as well as progressive loss of pancreatic function due to fibrosis of the parenchyma with consecutive endocrine and exocrine insufficiency.
Conclusions
The only success parameter of any treatment is the effective long-lasting pain relief and improvement in the quality of life. The surgical armamentarium includes simple drainage procedures, resections of different extents or a combination of both. Duodenum-preserving resection of the pancreas offers the best short-term outcome according to trials conducted so far. It has the benefit of combining the highest safety with the highest efficiency. Additionally, the extent of the operation can be adapted to the morphology of the individual patient.
Similar content being viewed by others
Introduction
Before being systematically described in 1946 by Comfort, the term ‘chronic pancreatitis’ (CP) has been used for a variety of pancreatic diseases without a generally accepted definition. In 1983, CP was defined as an ongoing inflammatory disease characterised by irreversible structural changes associated with abdominal pain and permanent loss of function. The considerable socioeconomic implications of the disease are caused by the persistent pain as well as frequent hospitalisation, often resulting in an inability to work and an early retirement of mostly young patients [53].
Alcohol consumption is the leading cause of CP in western countries (70–90%) [4], followed by cholelithiasis and autoimmune or individual genetic predisposition. High caloric intake of protein and fat, a lack of vitamins and trace elements, and especially smoking have been described as additional risk factors [78]. CP affects about eight new patients per 100,000 population per year in the USA, with a prevalence of 26.4 cases per 100,000 population [75]. Autopsy series, however, suggest a higher prevalence of 0.04% to 5%. The presentation of the disease peaks in patients between 35 and 55 years of age [4].
Different pathomorphological findings have been described in patients with CP. They can be found isolated, segmental or diffuse throughout the whole organ [2, 47, 78]. In general, the disease is characterised by an inflammatory process with progressive conversion of pancreatic parenchyma to fibrous tissue. Most commonly the calcifying or obstructive types of chronic pancreatitis are diagnosed that are characterised by a stenosis of the pancreatic duct and a consecutive prestenotic dilatation. Additionally, epithelial alterations, inflammatory periductal infiltrations, parenchymal atrophy, necrosis and fibrosis can be present [19, 46, 78].
The morphological and histological changes that characterise the disease are also extremely variable. Although the term pancreatitis implies inflammation, acute inflammatory changes are usually episodic and often absent. A change in microcirculatory perfusion and alterations of the epithelial permeability lead to an imbalance in the pancreatic juice, decreased fluids or bicarbonate secretion resulting in perilobular and intralobular fibrosis, ductal obstruction and periductal inflammation.
Natural course of chronic pancreatitis
At the time of onset of the disease, an at least moderate endocrine insufficiency is found in 8% of the patients. Approximately 80% will develop endocrine insufficiency in the long run [53, 57]. Usually, the natural course of CP is characterised by a progressive loss of endocrine and exocrine function due to a fibrotic transformation of the gland and persistent or recurrent pain [1, 78, 80].
The course of the disease starts with an initial period without any noticeable pain. The next stage is characterised by pain and exocrine preceding endocrine insufficiency. In the third stage, the pancreatic parenchyma is irreversibly converted to fibrous tissue with global insufficiency [19]. That 50–93% of patients will not become pain-free even after 10 years after the onset of the disease [53] has substantially challenged the paradigm that, in the long run, CP progression ‘burns out’ [3]. This belief was the main rationale for conservative approaches in the past. Additionally obstructive complications such as the formation of pancreatic pseudocysts as well as duodenal and common bile duct stenosis may be found.
Complications of chronic pancreatitis
CP is associated with several potential complications with more or less life-threatening potential that may occur in the natural course of the disease. Acute inflammatory episodes can lead to chronic pseudocysts or internal pancreatic fistula. An inflammatory mass in the pancreatic head can lead to gastric outlet obstruction. For the same reason, the intrapancreatic course of the common bile duct may be involved, eventually leading to cholestasis. Lastly, vascular derangements, especially thrombotic complications of the mesenterico-portal axis, can occur, resulting in localised (left-sided) or generalised portal hypertension or even cavernomatous transformation.
Common bile duct stenosis and duodenal obstruction
The development of common bile duct (CBD) stenosis is related to the close anatomical relationship of the distal CBD to the head of the pancreas. It is caused by an ongoing inflammatory process, scarring or fibrosis of the tissue of the pancreatic head. Pancreatic pseudocysts are a rare reason for compression of the common bile duct even though pseudocysts are commonly found in chronic pancreatitis. In hospitalised CP patients, the incidence of biliary stricture is about 5–9% and increases to up to 35% in surgical series [13, 33, 84, 88]. Clinical presentation of patients with stenosis ranges from asymptomatic cholestasis to severe cholangitis or complete CBD obstruction.
CBD strictures secondary to chronic pancreatitis facilitate decision-making in favour of surgery since the theoretical option of endoscopic dilatation/stenting, in the long run, usually fails. It is not recommended as definitive therapy, particularly with regard to the necessity of repeated endoscopic interventions due to infection, stent displacement or stent occlusion [11, 43]. However, interventional endoscopy may play a role in patients who are unfit for surgery or in the presence of cavernomatous transformation. While organ-preserving head resection with reinsertion of the CBD into the resection cavity preserves in such patients the gastroduodenal continuity, it is clearly associated with higher long-term morbidity because about 20% of patients will experience symptomatic, anastomotic stenosis at the reinsertion site [18]. Therefore, most centres favour pancreatoduodenectomy (PPPD or classical Whipple procedure) when CP is complicated by CBD stricture.
A gross cephalic mass, less frequently also pancreatic pseudocysts [87], may involve the duodenum and hereby eventually cause duodenal obstruction. A prospective surgical series reported on an incidence of duodenal obstruction (transient or permanent) of 12% [66]. Surgery is indicated for patients with failure to resolve the obstruction within 1–2 weeks of conservative therapy as this suggests the presence of an irreversible duodenal obstruction. Clinical symptoms of CP-related duodenal obstruction do not differ from that of other causes. A typical endoscopic finding is a concave-shaped extraluminal impression without mucosal involvement beginning at the descending portion of the duodenum.
It has been reported that the association of CBD stenosis and duodenal obstruction in CP patients accounts for 25% [87]. The therapeutic aim of surgery should be to eliminate the cause of the duodenal stricture, i.e. an enlarged pancreatic head or an encasement of the duodenum by inflammatory adhesions. Therefore, pancreaticoduodenectomy (PPPD or classical Whipple operation) is usually recommended.
Differentiating these inflammatory mass from malignancies may pre- and even intraoperatively be challenging. Approximately 10% of CP patients turn out to have pancreatic carcinoma [59]. Therefore, the knowledge of a CP history may even be misleading. It bears the risk that masses, which in the absence of CP would be clearly considered ‘suspicious’, are underestimated.
Internal pancreatic fistulas
Internal pancreatic fistulas result from a leakage of a pseudocyst or a disruption of the pancreatic duct. The incidence is low, but they are associated with significant morbidity and mortality [20, 70]. Most commonly, internal pancreatic fistulas present as pancreaticopleural fistula or pancreatic ascites with an overall incidence of approximately 4% in CP patients that is slightly higher (6–14%) when pancreatic pseudocysts are present. Mediastinal pseudocysts and pancreaticobronchial fistulas are rarely found [41, 76].
Pancreatic ascites is defined as a pancreatic fluid collection in the peritoneal cavity. The diagnosis is often difficult as pain or symptoms of a pancreatic disease may be absent. Especially in patients with alcohol abuse, the diagnosis may be confused with ascites due to cirrhosis and portal hypertension. Once an internal pancreatic fistula is suspected, laboratory testing of the concentration of amylase and lipase in the effusion should be performed. The level of amylase in the ascitic fluid is typically above 1,000 IU/L and the ascitic fluid to serum amylase ratio is approximately 6:1 [67, 82].
Evidence of internal pancreatic fistulas should entail the evaluation of the pancreatic duct morphology. Endoscopic retrograde pancreatography should be performed to localise the site of the leakage and to perform endoscopic therapy (transpapillary stenting of the pancreatic duct or sealing of the leak) [12]. Magnetic resonance cholangiopancreatography can accurately demonstrate the pancreatic duct and detect any abnormalities arising henceforth [74].
Conservative treatment is indicated in patients with mild to moderate ductal anatomic alterations [44, 54]; it consists of an application of somatostatin or octreotide together with diuretics and repeated paracentesis [63, 83]. The efficacy is reported to be 30% to 60% and the recurrence rate 15%; however, a mortality rate of 12% must be considered [31, 44].
Prior to endoscopic therapy in patients with persisting fistulas, a meticulous evaluation of ductal pathologies is mandatory. Ductal leakages into pseudocysts may be occluded by fibrin sealing. The knowledge that main pancreatic duct disruption often results from an increase in intraductal pressure is the underlying rationale to perform transpapillary endoscopic duct stenting in such patients [24, 50]. Indications for surgery include failure of conservative and endoscopic treatment and persistent or recurrent accumulation of ascites and/or sudden deterioration of clinical status.
Any surgical intervention has to address adequately right-sided, i.e. cephalic, alterations causing secondary, peripheral ductal hypertension which inhibits spontaneous closure of fistula and resolve the local problems to avoid redo surgery. Therefore, adequate drainage of the main pancreatic duct, decompression of the pseudocyst and, if necessary, a customised, organ-preserving pancreatic resection should be performed. The type of surgical intervention depends upon the ductal anatomy, site of the leakage from the pancreatic duct or pseudocyst, and further intraoperative findings [21].
Extrahepatic portal hypertension
Extrahepatic portal hypertension is a less common complication of chronic pancreatitis. It may be confined to either the superior mesenteric or splenic venous branch, but may also involve the whole spleno-mesenterico-portal axis [42]. It is defined as extrahepatic hypertension of the portal venous system in the absence of liver cirrhosis. The pathogenesis of extrahepatic portal hypertension in chronic pancreatitis is multifactorial.
The inflammatory process is capable of causing initial damage to vascular walls and may generate venous spasm, venous stasis and thrombosis [55]. Also, fibrosis of the pancreas can lead to progressive constriction of the spleno-mesenterico-portal axis. Other reasons are considerable pancreatic head enlargement or compression by pancreatic pseudocysts as well as inflammatory swelling of the gland [8, 56]. Extrahepatic portal hypertension alone is not an indication for surgical intervention in patients without further symptoms of chronic pancreatitis. Series evidencing an elevated risk to develop haemorrhage [9] to not exist, even though a potential risk of oesophageal or gastric varices exists [14]. Also, it has to be emphasised that these patients carry a considerably increased perioperative risk compared to patients without portal hypertension. If the varices start to bleed, therapeutic options include interventional measures such as sclerotherapy, variceal ligation and surgical portosystemic shunt procedures [8, 86].
Thrombosis of the portal vein with cavernous transformation
Pancreatitis is the most common extrahepatic, inflammatory condition that causes portal vein thrombosis and consecutive cavernous transformation. Patients with cavernous transformation were found to have a highly increased perioperative morbidity and mortality. A prolonged operation time and substantial intraoperative blood loss have to be expected. This makes the decision process highly complicated. The preoperative work-up should include a high-resolution CT to provide information on arterial blood supply and venous drainage. In the event of proven cavernous transformation, any decision-making in a symptomatic patient with failure of conservative and endoscopic treatment has to consider the considerable morbidity and mortality associated with upper abdominal surgery in such patients [10].
Indications for surgical intervention
The primary therapy for chronic pancreatitis is conservative, symptom-related treatment. Surgery has to be considered in patients with failure of conservative and endoscopic interventions. The most common indication for surgical treatment is intractable pain.
Further indications for surgical intervention are complications related to adjacent organs and non-resolving duodenal or common bile duct stenosis, endoscopically unmanageable pancreatic pseudocysts, especially if an underlying ductal pathology is evidenced, and internal pancreatic fistula intractable to conservative and interventional means [29, 52, 85]. Occasionally, the indication for surgical treatment of CP is the inability to exclude pancreatic cancer despite broad diagnostic work-up [69].
Few data exist on the timing of surgery in chronic pancreatitis. Nealon and co-workers found a delay of progressive functional impairment after surgery compared to conservative treatment and concluded that early operative drainage should be performed before the gland is functionally and morphologically irreversibly damaged [62]. Ihse and co-workers confirmed this notion suggesting that, in patients with obstructive chronic pancreatitis, early surgical treatment prior to an impairment of nutritional and metabolic status is of benefit [34].
Redo procedure
Little data exist concerning the benefits and risks of pancreatic redo surgery. Whereas primary pancreatic surgery requires expertise, it is even more demanding in redo procedures. The anatomy is altered and remarkable adhesions are commonly found. The access to the remnant of the pancreatic gland is difficult and the surrounding organs may be injured.
The indication for redo surgery in chronic pancreatitis are stenosis of the pancreaticojejunostomy, stenosis of the common bile duct or hepaticojejunostomy, respectively, and a persistent or recurrent pain syndrome (93%) due an ongoing inflammatory process in the remnant of the organ. Depending on the type of index procedure, the rate of recurrent pain ranges from 25% to 44% [32].
The causes for treatment failure are insufficient primary operation, recurrent inflammatory disease, anastomotic strictures (7%) or duodenal obstruction. Moreover, it was published that 5/350 patients that were primary operated on chronic pancreatitis needed redo surgery due to the development of a malignant tumour [72]. Completion cephalic pancreatectomy after previous left resection and PPPD after previous duodenum preserving resection of the pancreatic head (DPPHR) are the most common redo procedures. Perioperative outcome after redo surgery are controversial. Whereas in one small series, redo procedures after failure of index surgery had no mortality and reasonable morbidity of 33% [72], another series including patients that underwent redo procedures for benign and malignant diseases mortality was 6% [73]. Pain control was improved in 73% of patients after pancreatic redo surgery. It was shown that pancreatic redo surgery is technically demanding and therefore should be only performed in experienced centres [72, 73].
Controlled studies on the value of redo surgery after failure of index surgery do not exist. This concerns especially the impact of completion pancreatectomy after one or multiple previous operations in terms of an ultima ratio option to ensure quality of life.
Surgical procedures in treatment of chronic pancreatitis
Pancreatoduodenectomy
Partial pancreatoduodenectomy was initially described by Whipple–Kausch for treatment of malignant tumours of the pancreatic head. Its pylorus-preserving modification, as suggested by Longmire/Traverso, intends to reduce the bile reflux into the stomach after PD.
The major advantage of PD is the complete resection of the pancreatic head minimising the risk of disease recurrence originating from the pancreatic head. On the opposite, the major disadvantage of pancreatoduodenectomy is that the surrounding non-diseased organs (duodenum, CBD) are sacrificed with loss of the alimentary continuity. Due to the complex resection and reconstruction, the operation is technically demanding.
Total pancreatectomy
The role of total pancreatectomy is that of an ultimate salvage procedure for otherwise unmanageable pain. The spleen can rarely be protected. Additionally, total pancreatectomy is associated with high morbidity and mortality. The most common indication is a rescue pancreatectomy in patients with complications after pancreatic surgery (pancreatic fistula or anastomotic leakage) [78] or as a redo procedure for management of persistent or recurrent pain.
Distal pancreatectomy
Resection of the distal part of the pancreas is indicated for the management of severe complications localised in the pancreatic tail such as pseudoaneurysms or symptomatic pseudocysts that cannot be treated interventionally. Distal pancreatectomy is often associated with endocrine insufficiency. Additionally, the risk of simultaneous splenectomy must be considered. As the pancreatic head is often the pacemaker of chronic pancreatitis, distal resection is often ineffective in achieving pain control and crucial pathophysiologic mechanisms such as pancreatic duct hypertension and proximal stenosis are not addressed.
Simple drainage procedures
Coffey and Link were the first to describe ductal drainage procedures by opening the pancreatic main duct. Later on, DuVal and Zollinger independently inaugurated the decompression of the main pancreatic duct by resection of the pancreatic tail and retrograde drainage of the pancreatic duct via a termino-terminal or termino-lateral pancreaticojejunostomy. Puestow and Gillesby presented a modification including the decompression of the main pancreatic duct with resection of the pancreatic tail, splenectomy and longitudinal latero-lateral pancreaticojejunostomy. In 1960, Partington and Rochelle published a spleen-preserving longitudinal pancreaticojejunostomy without pancreatic tail resection [64] that represented the favoured surgical option for treatment of chronic pancreatitis for many years.
Duodenum-preserving resection of the pancreatic head
The first duodenum-preserving resection of the pancreatic head was introduced by Beger in 1980 [7]. A subtotal resection of the pancreatic head prior to a transsection of the gland above the portal vein is performed. The drainage of the pancreatic remnant is achieved by an end-to-end or end-to-side pancreaticojejunostomy using a Roux-en-Y loop.
A modification of the Beger procedure was suggested by Frey in 1985. A limited duodenum-preserving excision of the pancreatic head is performed by coring out the head of the pancreas, leaving a small cuff along the duodenal wall. Care is taken not to injure the common bile duct [28]. This procedure is combined with a longitudinal incision of the left-sided main pancreatic duct for optimal drainage. The latter aspect is comparable to the Partington–Rochelle procedure. The different extent of pancreatic head decompression and the fact that the pancreas is not divided over the superior mesenteric vein is the essential difference of the Beger and Frey procedures. A longitudinal pancreaticojejunostomy using a Roux-en-Y loop is used for drainage of the resection cavity of the pancreatic head and the opened, left-sided main duct.
The Hamburg procedure combines aspects of the Beger and Frey procedure. A subtotal resection of the pancreatic head including the uncinate process for optimal cephalic decompression is performed according to the Beger procedure while the transsection of the gland over the superior mesenteric portal vein is avoided. The excision is combined with a longitudinal V-shaped excision of the ventral aspect of the pancreas for optimal drainage of the main pancreatic duct in the tail of the pancreas. The operation is completed by a longitudinal pancreaticojejunostomy of the head, body and tail of the pancreas which is comparable to the Partington–Rochelle and Frey procedures.
The Berne procedure derives from a similar idea to combine the advantages of the Frey and the Beger procedures [30]. According to the principles of the Beger procedure, a deep duodenum-preserving resection of the pancreatic head for optimal decompression is performed, while the transsection of the gland over the superior mesenteric portal vein is avoided. In contrast to the Frey procedure and the Hamburg procedure, no drainage of the main pancreatic duct in the body and tail of the organ is performed. The drainage of the resection cavity of the pancreatic head is achieved by pancreaticojejunostomy using a Roux-en-Y loop as described by Beger.
V-shaped excision
For treatment of a rare subgroup of chronic pancreatitis that is characterised by a small pancreatic main duct (small-duct disease) characterised by a non-dilatated Wirsungian duct with narrowing or even what has been termed ‘pseudo-vanishing’, V-shaped incision was suggested by Izbicki and co-workers.
This procedure consists of a longitudinal deep and wide V-shaped excision of the ventral aspect of the pancreas with the tip of the V located at the main pancreatic duct and a longitudinal pancreaticojejunostomy with a Roux-en-Y loop [36].
Differential therapy and results
A surgical method has to be effective for the treatment of the underlying disease. The main success criterion of any treatment, especially of surgery in chronic pancreatitis, is to improve quality of life and to achieve pain relief or, at least, substantial pain reduction. Surgery should result in a decompression of the main pancreatic duct and resolve the complications concerning adjacent organs. Additionally, the intervention should be safe to perform and be associated with low mortality and morbidity. Besides pain management and an improvement of quality of life, an optimal procedure should ensure a low relapse rate and preserve a maximum of endocrine and exocrine function.
Major procedures for management of pain in chronic pancreatitis
Competing procedures for treatment of chronic pancreatitis are pancreatoduodenectomy (Whipple or PPPD) and duodenum-preserving pancreatic head resections of different extent (Beger, Frey, Hamburg and Berne). Several randomised controlled trials analysing their effectiveness have been performed. Simple drainage procedure, distal or total pancreatectomy, is recommended in special indications only and is discussed in a separate chapter.
The most relevant drainage procedure is the Partington–Rochelle procedure. Other modifications including a splenectomy, as performed in the Puestow procedure, increase the extent of the resection and possible complications and cannot longer be recommended. The standard drainage procedure was introduced by Partington–Rochelle, who suggested a longitudinal pancreaticojejunostomy preserving the spleen and pancreatic tail. Permanent pain relief was achieved in only 50–65% of the patients because of the usually present inflammatory mass in the pancreatic head including further pathologies, such as strictures, chronic pseudocysts, etc.
The thesis that the pancreatic head will be further impaired when patients undergo surgery, especially after resectional procedures, has been challenged by retrospective studies reporting only a minimal loss of pancreatic function. This finding was confirmed by two prospective studies, which reported that drainage procedures can even prevent the loss of pancreatic function. This illustrates the importance of halting the inflammatory process usually located in the pancreatic head to preserve endocrine and exocrine pancreatic function. The only suitable indication for a simple drainage procedure and for longitudinal pancreaticojejunostomy is in patients with a dilatated ductal diameter (>7 mm), or ‘chain of lakes’, without an inflammatory mass in the pancreatic head [48, 89]. It can be performed with low mortality and morbidity (approximately 3% and 20%, respectively). The major advantage of these procedures is that the maximum of pancreatic tissue is preserved. In most patients, the main pancreatic duct can be effectively drained. In short-term follow-up, pain relief is found in approximately 75% of the patients, but frequently it fails to provide long-lasting pain relief (up to 50%). However, it has to be stressed that randomised surgical trials comparing the Partington–Rochelle procedure with resectional operations of different extent [duodenum-preserving pancreatic head resection (Beger, Frey, Hamburg operation, Bern operation), Whipple resection, PPPD] have never been conducted. The reason for this lack of evidence is, most likely, that at least as reported by European centres, only few surgical patients experience ductal pathologies in the absence of disorders of their pancreatic head that is therefore usually also addressed during surgery in terms of a ‘one-stop-shopping therapy’ combining both resectional with draining aspects. In this context, one may refer to the Amsterdam trial reporting that most of the eligible chronic pancreatitis patients (79/118) that were screened for potential study participation had to be eventually excluded since they presented with radiographically evidenced pancreatic mass [17].
Therefore, despite this lack of clear evidence, one may hypothesise that the probable disadvantage of draining procedure is that a concomitant inflammatory pseudotumour in the pancreatic head including strictures and chronic pseudocysts is not addressed [48, 58, 65].
Duodenum-preserving pancreatic head resection, irrespective of which specific technique is performed, should combine resectional aspects (addressing the pancreatic head without scarifying the gastro-duodenal and bilioduodenal passage) with drainage aspects comparable to the Partington–Rochelle procedure (Frey/Hamburg). The deep subtotal excision (Beger/Hamburg/Berne) of the (enlarged) pancreatic head allows the resection of the fibrotic tissue and the altered neuronal structures. Also, DPPHR has to decompress the distal CBD if a complicating CBD stenosis is present. Compared to pancreatoduodenectomy, the advantage of all duodenum-preserving resections is that the physiological gastroduodenal passage and the common bile duct continuity are preserved [40]. Minimising the loss of normal pancreatic tissue, this procedure aims at a better pancreatic function.
The Beger procedure is reported to provide long-term pain relief in 75–95% of the patients [6, 15, 16, 27, 35–38]. The mortality rate ranges from 0% to 3% in experienced centres [16, 28, 38]. The morbidity rate was found to be 15–32% [14, 38, 49]. Beger et al. reported on a series of 388 patients of whom 91.3% were free of pain even after a median follow-up of 5.7 years [23, 68].
In a prospective trial, 56% of the patients were free of pain and 32% had substantial pain relief after Frey procedure. It was performed without mortality (<1%) and low morbidity (9–39%) [35, 38, 65]. After 5 years, 78% of the patients suffered from exocrine and 60% from endocrine insufficiency; 44% were professionally rehabilitated.
In the authors’ own experience, the Hamburg procedure, which basically combines a pancreatic head resection extent comparable with the Beger procedure and a ductal drainage left to the SMV/PV axis, is associated with mortality and morbidity rates of 0% and 19.6%, respectively. Eighty nine percent of the patients are free of pain in the follow-up; the body weight increase is significantly beneficial than after classic PPPD [51, 89]. Buechler and co-workers suggested the Berne procedure combining the Frey and Beger procedure [30]. This procedure has shown to be feasible, effective and safe with a mortality of 0–1% and a morbidity 20–23% [30]. Randomised trials comparing the Hamburg procedure with and the Bern procedure without left-sided ductal drainage have so far not been performed.
The rationale for pancreatoduodenectomy derives from the belief that the pancreatic head as the pacemaker of the disease is best treated by its entire removal [14, 39, 81]. Without radical surgery, so it is argued by advocators of Whipple resection, it is usually impossible to adequately drain not only the main pancreatic duct but also pancreatic ducts of second or third order. The results of PD concerning the aim of the treatment are excellent. An improvement in the quality of life as well as pain relief was found in short- and long-term follow-up in up to 90% of the patients [71]. Due to technical progress and growing experience, the procedure can be performed with low mortality (0–w5%) in experienced centres. However, morbidity of 20–53% is still high [5]. The extent of the resection leads to exocrine and often endocrine insufficiency.
Comparing classic Whipple procedure with pylorus-preserving partial pancreaticoduodenectomy, a significantly higher rate of pain and nausea and a lower quality of life was found [60], but the rate of pain control is similar. The major disadvantage of the procedure is that surrounding non-diseased organs are sacrificed. Nonetheless, it also plays an important role in the treatment of chronic pancreatitis. Any patient with a suspicion of pancreatic cancer should undergo partial pancreaticoduodenectomy to avoid hazardous redo operations due to inadequate index surgery.
Only two randomised controlled trials comparing the two different duodenum-preserving resections have been published so far. The first trial published by the authors in 1995 compared the Frey wit the Beger procedure. The perioperative mortality was 0 in both groups, while the morbidity rate was significantly lower after the Frey (9%) compared to 15% after the Beger procedure [38]. In an 8-year follow-up published by the authors in 2005, no significant differences concerning quality of life and pain score were found. The rate of endocrine and exocrine insufficiency and the reoperation rate were comparable in both groups [79].
The second RCT published by Koninger compares the Beger procedure and the Bern modification [49]. The duration of hospital stay and the operating time are shorter in the Bern modification. Moreover, a lower risk of anastomotic leakage was found after the Bern procedure, which is probably caused by the fact that in Beger’s procedure, two anastomoses must be performed, while only one is necessary in the Bern modification. No significant differences were found comparing the reoperation rates, complications and need for blood products. Comparing quality of life after both procedures were measured with a validated scoring tool (EORTC QLQ-PAN 26), better results were detected in the Bern group, while no difference was found when using the EORTC QLQ C30 tool [49].
Taken together, the Beger procedure is the only duodenum-preserving resection including a transsection of the pancreas above the superior mesenteric portal vein. Therefore, two pancreatic anastomoses are necessary. Compared to other procedures, it does not seem to offer substantial benefits. The Frey procedure, as originally introduced, leads only to a limited resection in the pancreatic head compared to the other modifications; it has been therefore defined as an ‘extended drainage’ operation. It may result in an incomplete decompression of the common bile duct in stenosis, persistent pain or recurrence.
During the last years, four prospective randomised trials comparing partial pancreatoduodenectomy and duodenum-preserving resection have been published. Amongst these studies, two report on long-term follow-up (>5 years). Farkas and co-workers found a similar rate of pain-free patients (86% vs. 83%) when comparing Beger procedure with pancreaticoduodenectomy. The operating time (142.5 ± 4.9 vs. 278.5 ± 6.9 min) and hospital stay (8.5 ± 0.9 vs. 13.8 ± 3.9 days) were longer in the pancreaticoduodenectomy group. Also, significant advantages in favour of the Beger group were detected concerning morbidity (0% vs. 30%) and increases in body weight (7.8 ± 0.9 vs. 3.2 ± 0.3 kg), while mortality and endocrine insufficiency were comparable [25, 26].
In another randomised trial presented by Klempa, the Beger procedure was superior in terms of postoperative endocrine status and of reconvalescence [45]. In a 24-month follow-up, Buechler and co-workers detected a significantly higher rate of pain-free patients after the Beger procedure (75%) compared to PPPD (40%). In support of this, patients in the Beger group had clear benefits in gaining body weight. In contrast, no significant differences were found in terms of morbidity, mortality and survival. The superiority of DPPHR over partial pancreatoduodenectomy in the short-term follow-up could no more be reproduced when the observation period was extended; after 14 years, quality of life and pain scores were nearly identical. This concerned also new-onset diabetes mellitus, enzyme substitution requirement and global health status. Only regarding the loss of appetite, favourable results in favour of the Beger procedure could still be detected [61].
Izbicki and co-workers presented the only randomised controlled trial comparing partial pancreatoduodenectomy with the Frey procedure for chronic pancreatitis. After 2 years, quality of life (85.7 vs. 75.1) and the pain score (6.1 vs. 18.1) were found to be superior in the Frey group. Also, the morbidity rate after the Frey procedure (19%) was significantly lower compared to pancreatoduodenectomy (53%). In contrast, in the long-term follow-up, the initially favourable results in favour of the Frey group concerning quality of life and pain scores tended to equal that of the partial pancreatoduodenectomy group. Consistently, no substantial difference was found in the rate of endocrine and exocrine insufficiency, the need for reoperations and late mortality [35, 77].
Summarising the key findings of the aforementioned trials, surgery offers good results in treatment of chronic pancreatitis. Quality of life improves substantially and pain control is achieved in the majority (~90%) of patients. Surgery does not appear to result in a substantial impairment of exocrine or endocrine function by any procedure compared to the natural course of the disease. In the short-term follow-up, duodenum-preserving resections are clearly beneficial compared to partial pancreatoduodenectomy, but in long-term follow-up, the results are roughly comparable. Not surprisingly, the different techniques of duodenum-preserving resections of the pancreatic head (Beger, Frey, Hamburg and Bern) offer similar results as all procedures yield a decompression of the pancreatic head as the pacemaker of the disease and effectively decompress the main pancreatic duct [22].
Special indications
A resection of the distal part of the pancreas is often associated with endocrine insufficiency. It only offers short pain relief. Therefore, left resection is usually obsolete for pain treatment. The only suitable indications are localised complications of the pancreatic tail such as chronic pseudocysts and pseudoaneurysms. The optimal closure of the pancreatic stump is discussed controversially.
The indication for V-shaped excision of the ventral aspect of the pancreas introduced by Izbicki is small-duct disease (sclerosing chronic pancreatitis) with a non-dilatated Wirsungian duct (narrowing or pseudo-vanishing) without an enlarged pancreatic head. Due to the absence of any ductal dilatation, such patients usually undergo extended resectional operations. The rationale of the procedure is that not only a parenchymal ‘compartment syndrome’ is addressed but also a better drainage of pancreatic ducts of second and third order is achieved. Excellent results were found after V-shaped excision with pain relief in 89% of the patients [89].
Also, mortality and morbidity of this procedure were found to be low (0% and 19.6%, respectively). A further advantage of this procedure is that the extent of the resection can be customised to the individual morphology of the pancreas [36]. In patients with an enlarged pancreatic head, V-shaped excision may be combined with a pancreatic head resection.
The indication for total pancreatectomy in chronic pancreatic is rare. The procedure should be avoided due to higher rates of mortality and morbidity, and complete endocrine and exocrine insufficiency. Its major indication is that of salvage surgery following one to several futile index operations.
Conclusion
Chronic pancreatitis is a common disease with consumption of alcohol and nicotine abuse as leading causes. It is usually characterised by an inflammatory mass located in the pancreatic head. The main success criterion of any treatment, irrespective of whether surgery, endotherapy or other modalities are applied, is the achievement of primary pain relief and an improvement in quality of life. Surgery should maintain as much exocrine and endocrine function as possible. Pancreatic surgery is technically demanding and is associated with potential life-threatening complications. Therefore, it should be left to experts in high-volume hospitals to minimise mortality and morbidity.
For the majority of patients, duodenum-preserving resection of the pancreas seems to be the ideal operation. Although the long-term results of partial pancreatoduodenectomy are comparable, organ-preserving surgery combines high safety with high efficacy and is associated with the best short-term outcome.
References
Adler DG, Lichtenstein D, Baron TH, Davila R, Egan JV, Gan SL, Qureshi WA, Rajan E, Shen B, Zuckerman MJ, Lee KK, VanGuilder T, Fanelli RD (2006) The role of endoscopy in patients with chronic pancreatitis. Gastrointest Endosc 63(7):933–937
Ammann RW (1997) A clinically based classification system for alcoholic chronic pancreatitis: summary of an international workshop on chronic pancreatitis. Pancreas 14:215–221
Ammann RW, Akovbiantz A, Largiader F, Schueler G (1984) Course and outcome of chronic pancreatitis. Longitudinal study of a mixed medical–surgical series of 245 patients. Gastroenterology 86:820–888
Ammann RW, Mullhaupt B (2007) Do the diagnostic criteria differ between alcoholic and nonalcoholic chronic pancreatitis? J Gastroenterol 42(Suppl 17):118–126
Bachmann K, Mann O, Izbicki JR, Strate T (2008) Chronic pancreatitis—a surgeons' view. Med Sci Monit 14(11):RA198–RA205
Beger HG, Buechler M (1990) Duodenum preserving resection of the head of the pancreas in chronic pancreatitis with inflammatory mass in the head. World J Surg 14:83–87
Beger HG, Witte C, Krautzberger W, Bittner R (1980) Experiences with duodenum-sparing pancreas head resection in chronic pancreatitis. Chirurg 51:303–307
Bernades P, Baetz A, Levy P, Belghiti J, Menu Y, Fekete F (1992) Splenic and portal venous obstruction in chronic pancreatitis. A prospective longitudinal study of a medical–surgical series of 266 patients. Dig Dis Sci 37:340–346
Bloechle C, Busch C, Tesch C, Nicolas V, Binmoeller KF, Soehendra N, Izbicki JR (1997) Prospective randomized study of drainage and resection on non-occlusive segmental portal hypertension in chronic pancreatitis. Br J Surg 84:477–482
Bockhorn M, Gebauer F, Bogoevski D, Molmenti E, Cataldegirmen G, Vashist YK, Yekebas EF, Izbicki JR, Mann O (2010) Chronic pancreatitis complicated by cavernous transformation of the portal vein: contraindication to surgery? Surgery. doi:10.1016/j.surg.2010.06.011
Born P, Rosch T, Bruhl K, Ulm K, Sandschin W, Frimberger E, Allescher H, Classen M (1998) Long-term results of endoscopic treatment of biliary duct obstruction due to pancreatic disease. Hepatogastroenterology 45(21):833–839
Bracher GA, Manocha AP, DeBanto JR, Gates LK Jr, Slivka A, Whitcomb DC, Bleau BL, Ulrich CD, Martin SP (1999) Endoscopic pancreatic duct stenting to treat pancreatic ascites. Gastrointest Endosc 49(6):710–715
Bradley EL (1987) Long-term results of pancreatojejunostomy in patients with chronic pancreatitis. Am J Surg 153:207–213
Buchler MW, Friess H, Muller MW, Wheatley AM, Beger HG (1995) Randomized trial of duodenum-preserving pancreatic head resection versus pylorus-preserving Whipple in chronic pancreatitis. Am J Surg 169(1):65–69
Buechler M, Friess H, Isenmann R, Bittner R, Beger HG (1993) Duodenum-preserving resection of the head of the pancreas: the Ulm experience. In: Beger HG, Buechler M, Malfertheimer P (eds) Standards in pancreatic surgery. Springer, Berlin, pp 436–449
Buechler MW, Friess H, Bittner R, Roscher R, Krautzberger W, Mueller MW, Malfertheiner P, Beger HG (1997) Duodenum-preserving pancreatic head resection: long-term results. J Gastrointest Surg 1:13–19
Cahen DL, Gouma DJ, Nio Y, Rauws EA, Boermeester MA, Busch OR, Stoker J, Lameris JS, Dijkgraaf MG, Huibregtse K, Bruno MJ (2007) Endoscopic versus surgical drainage of the pancreatic duct in chronic pancreatitis. N Engl J Med 356(7):676–684
Cataldegirmen G, Bogoevski D, Mann O, Kaifi JT, Izbicki JR, Yekebas EF (2008) Late morbidity after duodenum-preserving pancreatic head resection with bile duct reinsertion into the resection cavity. Br J Surg 95(4):447–452
Chari ST (2007) Chronic pancreatitis: classification, relationship to acute pancreatitis, and early diagnosis. J Gastroenterol 42(Suppl 17):58–59
Chebli JM, Gaburri PD, De Souza AF, Ornellas AT, Martins Junior EV, Chebli LA, Felga GE, Pinto JR (2004) Internal pancreatic fistulas: proposal of a management algorithm based on a case series analysis. J Clin Gastroenterol 38(9):795–800
da Cunha JE, Machado M, Bacchella T, Penteado S, Mott CB, Jukemura J, Pinotti HW (1995) Surgical treatment of pancreatic ascites and pancreatic pleural effusions. Hepatogastroenterology 42(5):748–751
Diener MK, Rahbari NN, Fischer L, Antes G, Buchler MW, Seiler CM (2008) Duodenum-preserving pancreatic head resection versus pancreatoduodenectomy for surgical treatment of chronic pancreatitis: a systematic review and meta-analysis. Ann Surg 247(6):950–961
Duffy JP, Delano MJ, Reber HA (2002) Pancreatic surgery. Curr Opin Gastroenterol 18(5):568–573
Eckhauser F, Raper SE, Knol JA, Mulholland MW (1991) Surgical management of pancreatic pseudocysts, pancreatic ascites, and pancreaticopleural fistulas. Pancreas 6(Suppl 1):S66–S75
Farkas G, Leindler L, Daroczi M, Farkas G Jr (2008) Long-term follow-up after organ-preserving pancreatic head resection in patients with chronic pancreatitis. J Gastrointest Surg 12(2):308–312
Farkas G, Leindler L, Daroczi M, Farkas G Jr (2006) Prospective randomised comparison of organ-preserving pancreatic head resection with pylorus-preserving pancreaticoduodenectomy. Langenbecks Arch Surg 391(4):338–342
Frey CF, Amikura K (1994) Local resection of the head of the pancreas combined with longitudinal pancreaticojejunostomy in the management of patients with chronic pancreatitis. Ann Surg 220:492–507
Frey CF, Smith GJ (1987) Description and rationale of a new operation for chronic pancreatitis. Pancreas 2:701–707
Frey CF, Suzuki M, Isaji S (1990) Treatment of chronic pancreatitis complicated by obstruction of the common bile duct or duodenum. World J Surg 14:59–69
Gloor B, Friess H, Uhl W, Buchler MW (2001) A modified technique of the Beger and Frey procedure in patients with chronic pancreatitis. Dig Surg 18:21–25
Gomez-Cerezo J, Barbado CA, Suarez I, Soto A, Rios JJ, Vazquez JJ (2003) Pancreatic ascites: study of therapeutic options by analysis of case reports and case series between the years 1975 and 2000. Am J Gastroenterol 98(3):568–577
Howard TJ, Browne JS, Zyromski NJ, Lavu H, Baker MS, Shen C, Madura JA (2008) Mechanisms of primary operative failure and results of remedial operation in patients with chronic pancreatitis. J Gastrointest Surg 12(12):2087–2095
Huizinga WK, Thomson SR, Spitaels JM, Simjee AE (1992) Chronic pancreatitis with biliary obstruction. Ann R Coll Surg Engl 74(2):119–123
Ihse I, Borch K, Larsson J (1990) Chronic pancreatitis: results of operations for relief of pain. World J Surg 14(1):53–58
Izbicki JR, Bloechle C, Broering DC, Knoefel WT, Kuechler T, Broelsch CE (1998) Extended drainage versus resection in surgery for chronic pancreatitis—prospective randomized trial comparing the longitudinal pancreaticojejunostomy combined with local pancreatic head excision with the pylorus preserving pancreatoduodenectomy. Ann Surg 228:771–779
Izbicki JR, Bloechle C, Broering DC, Kuechler T, Broelsch CE (1998) Longitudinal V-shaped excision of the ventral pancreas for small duct disease in severe chronic pancreatitis. Ann Surg 227:213–219
Izbicki JR, Bloechle C, Knoefel WT, Binmoeller KF, Soehendra N, Broelsch CE (1997) Drainage versus Resektion in der chirurgischen Therapie der chronischen Kopfpankreatitis: eine randomisierte Studie. Chirurg 68:369–377
Izbicki JR, Bloechle C, Knoefel WT, Kuechler T, Binmoeller KF, Broelsch CE (1995) Duodenum preserving resections of the head of the pancreas in chronic pancreatitis—a prospective randomized trial. Ann Surg 221:350–358
Izbicki JR, Bloechle C, Knoefel WT, Rogiers X, Kuechler T (1999) Surgical treatment of chronic pancreatitis and quality of life after operation. Surg Clin North Am 79:913–944
Izbicki JR, Bloechle C, Knoefel WT, Wilker DK, Dornschneider G, Seifert H, Passlick B, Rogiers X, Busch C, Broelsch CE (1994) Complications of adjacent organs in chronic pancreatitis managed by duodenum-preserving resection of the head of the pancreas. Br J Surg 81:1351–1355
Izbicki JR, Wilker DK, Waldner H, Rueff FL, Schweiberer L (1989) Thoracic manifestations of internal pancreatic fistulas: report of five cases. Am J Gastroenterol 84(3):265–271
Izbicki JR, Yekebas EF, Strate T, Eisenberger CF, Hosch SB, Steffani K, Knoefel WT (2002) Extrahepatic portal hypertension in chronic pancreatitis: an old problem revisited. Ann Surg 236:82–89
Kahl S, Zimmermann S, Genz I, Glasbrenner B, Pross M, Schulz HU, Mc ND, Schmidt U, Malfertheiner P (2003) Risk factors for failure of endoscopic stenting of biliary strictures in chronic pancreatitis: a prospective follow-up study. Am J Gastroenterol 98(11):2448–2453
Kaman L, Behera A, Singh R, Katariya RN (2001) Internal pancreatic fistulas with pancreatic ascites and pancreatic pleural effusions: recognition and management. ANZ J Surg 71(4):221–225
Klempa I, Spatny M, Menzel J, Baca I, Nustede R, Stoeckmann F, Arnold W (1995) Pankreasfunktion und Lebensqualität nach Pankreaskopfresektion bei der chronischen Pankreatitis. Chirurg 66:350–359
Kloppel G, Maillet B (1993) Pathology of acute and chronic pancreatitis. Pancreas 8(6):659–670
Kloppel G, Maillet B (1992) The morphological basis for the evolution of acute pancreatitis into chronic pancreatitis. Virchows Arch A Pathol Anat Histopathol 420(1):1–4
Knoefel WT, Eisenberger CF, Strate T, Izbicki JR (2002) Optimizing surgical therapy for chronic pancreatitis. Pancreatology 2:379–385
Koninger J, Seiler CM, Sauerland S, Wente MN, Reidel MA, Muller MW, Friess H, Buchler MW (2008) Duodenum-preserving pancreatic head resection—a randomized controlled trial comparing the original Beger procedure with the Berne modification (ISRCTN No. 50638764). Surgery 143(4):490–498
Kozarek RA, Ball TJ, Patterson DJ, Raltz SL, Traverso LW, Ryan JA, Thirlby RC (1997) Transpapillary stenting for pancreaticocutaneous fistulas. J Gastrointest Surg 1(4):357–361
Kutup A, Vashist Y, Kaifi JT, Yekebas EF, Izbicki JR (2010) For which type of chronic pancreatitis is the "Hamburg procedure" indicated? J Hepatobiliary Pancreat Sci 17(6):758–762
Lankisch PG, Andren-Sandberg A (1993) Standards for the diagnosis of chronic pancreatitis and for the evaluation of treatment. Int J Pancreatol 14:205–212
Lankisch PG, Happe-Loehr A, Otto J, Creutzfeldt W (1993) Natural course in chronic pancreatitis. Pain, exocrine and endocrine pancreatic insufficiency and prognosis of the disease. Digest 54:148–155
Lipsett PA, Cameron JL (1992) Internal pancreatic fistula. Am J Surg 163(2):216–220
Little AG, Moossa AR (1981) Gastrointestinal hemorrhage from left-sided portal hypertension. An unappreciated complication of pancreatitis. Am J Surg 141(1):153–158
Malka D, Hammel P, Levy P, Sauvanet A, Ruszniewski P, Belghiti J, Bernades P (1998) Splenic complications in chronic pancreatitis: prevalence and risk factors in a medical–surgical series of 500 patients. Br J Surg 85(12):1645–1649
Malka D, Hammel P, Sauvanet A, Rufat P, O'Toole D, Bardet P, Belghiti J, Bernades P, Ruszniewski P, Levy P (2000) Risk factors for diabetes mellitus in chronic pancreatitis. Gastroenterology 119:1324–1332
Markowitz JS, Rattner DW, Warshaw AL (1994) Failure of symptomatic relief after pancreaticojejunal decompression for chronic pancreatitis. Strategies for salvage. Arch Surg 129:374–379
Mayerle J, Lerch MM (2007) Is it necessary to distinguish between alcoholic and nonalcoholic chronic pancreatitis? J Gastroenterol 42(Suppl 17):127–130
Mobius C, Max D, Uhlmann D, Gumpp K, Behrbohm J, Horvath K, Hauss J, Witzigmann H (2007) Five-year follow-up of a prospective non-randomised study comparing duodenum-preserving pancreatic head resection with classic Whipple procedure in the treatment of chronic pancreatitis. Langenbecks Arch Surg 392(3):359–364
Muller MW, Friess H, Martin DJ, Hinz U, Dahmen R, Buchler MW (2008) Long-term follow-up of a randomized clinical trial comparing Beger with pylorus-preserving Whipple procedure for chronic pancreatitis. Br J Surg 95(3):350–356
Nealon WH, Thompson JC (1993) Progressive loss of pancreatic function in chronic pancreatitis is delayed by main pancreatic duct decompression. Ann Surg 217:458–468
Oktedalen O, Nygaard K, Osnes M (1990) Somatostatin in the treatment of pancreatic ascites. Gastroenterology 99(5):1520–1521
Partington PF, Rochelle REL (1960) Modified Puestow procedure for retrograde drainage of the pancreatic duct. Ann Surg 152:1037–1043
Pessaux P, Kianmanesh R, Regimbeau JM, Sastre B, Delcenserie R, Sielezneff I, Arnaud JP, Sauvanet A (2006) Frey procedure in the treatment of chronic pancreatitis: short-term results. Pancreas 33(4):354–358
Prinz RA, Aranha GV, Greenlee HB (1985) Combined pancreatic duct and upper gastrointestinal and biliary tract drainage in chronic pancreatitis. Arch Surg 120(3):361–366
Runyon BA (1987) Amylase levels in ascitic fluid. J Clin Gastroenterol 9(2):172–174
Ryder NM, Reber HA (2000) Pancreatic surgery. Curr Opin Gastroenterol 16(5):426–430
Saeger HD, Schwall G, Trede M (1993) Standard Whipple in chronic pancreatitis. In: Beger HG, Buechler M, Malfertheimer P (eds) Standards in pancreatic surgery. Springer, Berlin, pp 385–391
Saps M, Slivka A, Khan S, Meza MP, Goyal A, Di LC (2003) Pancreatic ascites in an infant: lack of symptoms and normal amylase. Dig Dis Sci 48(9):1701–1704
Schnelldorfer T, Lewin DN, Adams DB (2006) Reoperative surgery for chronic pancreatitis: is it safe? World J Surg 30(7):1321–1328
Seelig MH, Chromik AM, Weyhe D, Muller CA, Belyaev O, Mittelkotter U, Tannapfel A, Uhl W (2007) Pancreatic redo procedures: to do or not to do—this is the question. J Gastrointest Surg 11(9):1175–1182
Seelig MH, Janot M, Chromik AM, Herzog T, Belyaev O, Weyhe D, Meurer K, Meiser A, Tannapfel A, Uhl W (2009) Redo-surgery following curative resection of pancreatic carcinoma: the difference between true and suspected recurrence. Dig Surg 26(3):222–228
Soto JA, Barish MA, Yucel EK, Clarke P, Siegenberg D, Chuttani R, Ferrucci JT (1995) Pancreatic duct: MR cholangiopancreatography with a three-dimensional fast spin-echo technique. Radiology 196(2):459–464
Steer ML, Waxman I, Freedman S (1995) Chronic pancreatitis. N Engl J Med 332(22):1482–1490
Stenger AM, Knoefel WT, Dahmen U, Blochle C, Izbicki JR (1998) Pancreatico-bronchial fistula with communication to a pseudoaneurysm of the arteria lienalis as a rare complication in chronic pancreatitis. Z Gastroenterol 36(12):1047–1051
Strate T, Bachmann K, Busch P, Mann O, Schneider C, Bruhn JP, Yekebas E, Kuechler T, Bloechle C, Izbicki JR (2008) Resection vs drainage in treatment of chronic pancreatitis: long-term results of a randomized trial. Gastroenterology 134(5):1406–1411
Strate T, Knoefel WT, Yekebas E, Izbicki JR (2003) Chronic pancreatitis: etiology, pathogenesis, diagnosis, and treatment. Int J Colorectal Dis 18(2):97–106
Strate T, Taherpour Z, Bloechle C, Mann O, Bruhn JP, Schneider C, Kuechler T, Yekebas E, Izbicki JR (2005) Long-term follow-up of a randomized trial comparing the Beger and Frey procedures for patients suffering from chronic pancreatitis. Ann Surg 241(4):591–598
Strate T, Yekebas E, Knoefel WT, Bloechle C, Izbicki JR (2002) Pathogenesis and the natural course of chronic pancreatitis. Eur J Gastroenterol Hepatol 14:929–934
Traverso LW, Tompkins RK, Urrea PT, Longmire WP Jr (1979) Surgical treatment of chronic pancreatitis. Twenty-two years' experience. Ann Surg 190(3):312–319
Uchiyama T, Yamamoto T, Mizuta E, Suzuki T (1989) Pancreatic ascites—a collected review of 37 cases in Japan. Hepatogastroenterology 36(4):244–248
Uhl W, Buchler MW, Malfertheiner P, Beger HG, Adler G, Gaus W (1999) A randomised, double blind, multicenter trial of octreotide in moderate to severe acute pancreatitis. Gut 45:97–104
Vijungco JD, Prinz RA (2003) Management of biliary and duodenal complications of chronic pancreatitis. World J Surg 27(11):1258–1270
Warshaw AL (1984) Pain in chronic pancreatitis—patients, patience, and the impatient surgeon. Gastroenterology 86:987–989
Warshaw AL, Jin GL, Ottinger LW (1987) Recognition and clinical implications of mesenteric and portal vein obstruction in chronic pancreatitis. Arch Surg 122:410–445
Warshaw AL, Rattner DW (1985) Timing of surgical drainage for pancreatic pseudocyst. Clinical and chemical criteria. Ann Surg 202(6):720–724
Wislooff F, Jakobsen J, Osnes M (1982) Stenosis of the common bile duct in chronic pancreatitis. Br J Surg 69(1):52–54
Yekebas EF, Bogoevski D, Honarpisheh H, Cataldegirmen G, Habermann CR, Seewald S, Link BC, Kaifi JT, Wolfram L, Mann O, Bubenheim M, Izbicki JR (2006) Long-term follow-up in small duct chronic pancreatitis: a plea for extended drainage by "V-shaped excision" of the anterior aspect of the pancreas. Ann Surg 244(6):940–946
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bachmann, K., Izbicki, J.R. & Yekebas, E.F. Chronic pancreatitis: modern surgical management. Langenbecks Arch Surg 396, 139–149 (2011). https://doi.org/10.1007/s00423-010-0732-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-010-0732-0