Abstract
Purpose
To compare the perceptual responses and interleukin-6 (IL-6) concentration following rectal temperature-matched dry heat exposure (DH) and hot water immersion (HWI).
Methods
Twelve healthy young adults (BMI 23.5 ± 3.6 kg/m2; age: 25.8 ± 5.7 years) underwent 3 trials in randomised order: DH (air temperature 68.9 °C), HWI (water temperature 37.5 °C), and thermoneutral dry exposure (CON, air temperature 27.3 °C). Blood samples to determine IL-6 plasma concentration were collected; basic affect and thermal comfort, rectal and skin temperature (Tskin) were assessed throughout the intervention.
Results
Rectal temperature (Trec) did not differ between DH (end temperature 38.0 ± 0.4 °C) and HWI (37.9 ± 0.2 °C, P = 0.16), but was higher compared with CON (37.0 ± 0.3 °C; P ≤ 0.004). Plasma IL-6 concentration was similar after DH (pre to post: 0.8 ± 0.5 to 1.4 ± 1.5 pg·ml−1) and HWI (0.5 ± 0.2 to 0.9 ± 0.6 pg·ml−1; P = 0.46), but higher compared with CON (0.6 ± 0.5 to 0.6 ± 0.4 pg·ml−1; P = 0.01). At the end of the intervention, basic affect and thermal comfort were most unfavourable during DH (Basic affect; DH: − 0.7 ± 2.9, HWI: 0.8 ± 1.9, CON 1.9 ± 1.9, P ≤ 0.004; Thermal comfort; 2.6 ± 0.8, HWI: 1.4 ± 0.9 and CON: 0.2 ± 0.4; P ≤ 0.004). Mean Tskin was highest for DH, followed by HWI, and lowest for CON (DH: 38.5 ± 1.3 °C, HWI: 36.2 ± 0.5 °C, CON: 31.6 ± 0.7 °C, P < 0.001).
Conclusion
The IL-6 response did not differ between DH and HWI when matched for the elevation in Trec. However, thermal comfort was lower during DH compared to HWI, which may be related to the higher Tskin during DH.
Similar content being viewed by others
Introduction
Acute as well as regular, repeated exposure to heat induces a range of physiological adaptations (Ely et al. 2019). For example, a single passive heating session acutely increases arterial blood flow, energy expenditure and the circulating concentration of inflammatory markers (McCarty et al. 2009; Yildirim et al. 2010; Hoekstra et al. 2020; Brunt and Minson 2021). Moreover, the circulating concentration of the pleiotropic cytokine interleukin (IL)-6 has been shown to increase following a single passive heating session of varying durations and temperatures (Laing et al. 2008; Faulkner et al. 2017; Hashizaki et al. 2018; Hoekstra et al. 2018, 2021; Mansfield et al. 2021). Evidence from infusion studies indicates that acute transient elevation of IL-6 concentration stimulates the release of anti-inflammatory cytokines such as IL-10 and IL-1 receptor antagonist; leading to an anti-inflammatory milieu (Steensberg et al. 2003). Based on this notion, it is suggested that the positive long-term health adaptations following repeated exposure to passive heating (i.e. heat therapy) may be partly explained by the acute inflammatory response occurring following each passive heating session and the subsequent reduction in the concentration of basal pro-inflammatory markers such as tumour necrosis factor-α and C-reactive protein (Petersen and Pedersen 2005; Oyama et al. 2013; Leicht et al. 2015; Ely et al. 2017; Simmons et al. 2017; Hoekstra et al. 2018). Furthermore, blocking IL-6 signalling through tocilizumab abolishes the positive effects of exercise training on adipose tissue mass; suggesting an additional role for repeated transient elevations in IL-6 concentration in lipid metabolism. Thus, in line with the concept of hormesis (Martin et al. 2010), repeated transient elevations of IL-6 concentration appear beneficial for cardiometabolic health, and can be instigated by heat therapy.
Heat therapy is accessible and low-cost, while it has minimal side-effects compared to pharmaceutical agents (Simkin and Bolding 2004). Common modes of heat exposure include dry heat (DH) exposure (e.g., Finnish sauna, far infrared sauna, Waon therapy) and hot water immersion (HWI) (Brunt and Minson 2021). In all modes, passive heat stress raises skin (Tskin) and rectal temperature (Trec), and activates the autonomic nervous system, increasing heart rate (HR), respiration and sweat rate (Kukkonen-Harjula et al. 1989; Jezova et al. 2007; Kikuchi et al. 2013). The specific mode of heating influences the temperature to which the skin is directly exposed. The temperature in a sauna (~ 80–100 °C) (Helamaa and Aikas 1988), exposing the body to DH, differs substantially to the water temperature in typical HWI protocols (~ 38–42 °C) (Cheung and Sleivert 2004; Goto et al. 2011; Hoekstra et al. 2018). As the heat transfer is 24 times higher in water than in air (Nadel 1977), substantially lower water temperatures can achieve a similar increase in Trec (Brunt and Minson 2021). However, the higher environmental temperature during DH is likely to induce a higher rise in Tskin when compared with HWI (Campbell et al. 2022). A difference in Tskin between DH and HWI may affect peripheral muscle temperature (Chiesa et al. 2015), which may hence affect inflammatory responses, as a high skeletal muscle temperature can stimulate muscle IL-6 release (Welc et al. 2012). Although an acute increase in circulating IL-6 concentration has been observed for both DH and HWI (Kaldur et al. 2016; Hoekstra et al. 2018), it is unknown how the inflammatory response compares between both heating modes.
Given that Tskin is a major determinant of thermal comfort (Frank et al. 1999), perceptual responses may differ between DH and HWI. Furthermore, HWI may cause skin maceration and skin softening (An et al. 2019), which may also affect perception. On the other hand, sweaty clothing in DH may also cause discomfort (Jiang and Wang 2020). These factors are highly relevant when prescribing heat therapy regimens, as individuals are more likely to repeat behaviour they find comfortable and from which they derive pleasure (Jung et al. 2014; Hoekstra et al. 2018). At the same time, the health effects of heat therapy appear to be dose-dependent (Zaccardi et al. 2017; Kunutsor et al. 2018), exemplified by a 40% lower risk of all-cause mortality in frequent compared to infrequent sauna users (Laukkanen et al. 2015). Thus, insight into factors promoting adherence to heat therapy are important for the success of future long-term interventions.
The aim of this study was therefore to compare the inflammatory and perceptual responses to rectal temperature-matched DH and HWI. It was hypothesised that (1) DH induces a larger rise in Tskin than HWI, (2) due to the higher Tskin in DH, the acute elevation of IL-6 is higher in DH compared with HWI and (3) perceptions during HWI are more positive than in DH.
Methods
Participants
Participants were 12 healthy young adults (3 females, 9 males; age: 25.8 ± 5.7 years; height: 177.2 ± 9.3 cm; weight: 74.3 ± 14.2 kg; self-reported structured exercise: 4.0 ± 3.1 h/week). All participants in the study filled out a standard health questionnaire used throughout the Department at Loughborough University to ensure no contraindications for heat therapy were present. Further exclusion criteria were smoking, the use of anti-inflammatory medication and engaging in structured exercise for more than 8 h/week. In line with the Declaration of Helsinki, participants gave written informed consent after being informed of the study procedures, approved by Loughborough University’s ethics committee (code: 1308).
Experimental design
Participants visited the laboratory to undergo three experimental conditions in a randomised order: DH exposure, HWI and a control condition (CON) (Fig. 1). During DH, participants maintained a supine position in the Cocoon POD (Wellness USA, Minneapolis, Minnesota, USA). The entire body except the head was exposed to DH. The Cocoon POD setting used was ‘high-hyper’ (the highest temperature setting), resulting in an air temperature of 68.9 ± 3.2 °C and 7.8 ± 2.7% humidity. For HWI, the water temperature was set at 37.5 °C and maintained manually. Participants maintained a seating position and were immersed in water up to the neck. The water temperature was chosen with the aim to match the Trec rise observed in the Cocoon POD, as determined during pilot testing. During CON, participants rested in the Cocoon POD at a thermoneutral temperature (27.3 ± 1.4 °C and 39.4 ± 4.8% humidity). The temperature and humidity in the Cocoon POD were measured using an I-button (Homechip Ltd, Milton Keynes, UK) attached to the roof of the device, at the level of the participants’ chest. All visits began between 11 am and 1 pm to control for potential circadian rhythm variation in any of the outcome measures. Visits were separated by a minimum of 48 h and were completed within 60 days. Participants were asked to avoid exercise, caffeine, and alcohol intake the day before the laboratory visit. In addition, they kept a 24-h dietary record before the first visit and replicated that diet for subsequent visits.
At the start of every session, the following devices were applied and worn throughout the entire trial: (1) a rectal probe (YSI Precision ™ 4000A Thermometer, Ohio, USA) inserted ~ 10 cm past the anal sphincter for the measurement of Trec, (2) six Tskin sensors (I-buttons, Homechip Ltd, Milton Keynes, UK) placed on the skin (forehead, cheek, chest, arm, thigh, calf) to measure Tskin, (3) a Polar heart rate monitor (Polar, Kempele, Finland) to monitor HR. Body mean Tskin was calculated as a weighed mean of the arm, chest, thigh and calf (Ramanathan 1964):
Aside from the absolute values, the rate of increase in mean Tskin was calculated from baseline to peak Tskin. During the intervention, expired air was collected every 30 min and perceptual responses were reported every 15 min. Expired air was collected in Douglas bags over a three-minute period. Expired air was analysed using a Servomex 1440 gas analyser (Servomex Ltd, Crowborough, UK) to obtain oxygen uptake and carbon dioxide production rates to determine energy expenditure (de Weir 1949). The following perceptual responses were reported using visual analogue scales: thermal sensation (1 very cold–9 very hot) (Epstein and Moran 2006), thermal comfort (0 comfortable to + 4 very uncomfortable) (Gagge and Nishi 1976), skin wetness perception (face and other parts of the body; − 3 very dry to + 3 very wet) (Filingeri et al. 2015), basic affect (i.e., ‘how do you feel at this moment in time?’; − 5 very bad to + 5 very good) (Hardy and Rejeski 1989).
Following the measurement of height and nude body mass, participants rested in a seated position for 30 min. After that, physiological and temperature measurements were obtained, while participants also reported their thermal sensation, thermal comfort, basic affect and skin wetness perception. The participants then entered the Cocoon POD (DH) or water (HWI) for 60 min with measurements taken every 15 min. During all visits, water intake was allowed ad libitum. At the end of 60 min, nude body mass was recorded again for the estimation of sweat loss, taking any water consumed into account. Participants then rested in a seated position for a further 60 min. They were allowed to perform non-strenuous tasks such as reading or working on a laptop. Further measurements were obtained 30 and 60 min after the session. At the end of each visit, participants were asked to rate their enjoyment of the intervention on a 20 cm visual analogue scale (0—not pleasant to 20—very pleasant). After completing all three trials, participants were asked to select their favourite trial, provide a fondness rating (on a scale from 1 least liked to 9 most liked) for each session, and explain why they preferred the chosen session.
Biochemical analysis
Blood was collected in tri-potassium EDTA monovettes, which were spun at 3500 rpm and 4 °C for 10 min. The plasma was divided into aliquots and stored at − 80 °C until batch analysis. Plasma IL-6 was measured using a high sensitivity enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer’s instructions (R&D Systems, Abingdon, UK), using a microplate reader (Varioskan Flash, ThermoScientific, Waltham, US). The intra-assay coefficient of variation was 6.4%. Haematocrit, determined in duplicate using a microcentrifuge, and haemoglobin concentration, determined by a Yumizen H500 automated analyser (Horiba Medical, Montpellier, France) were used to correct IL-6 concentrations for changes in plasma volume (Dill and Costill 1974).
Statistical analysis
The statistical software SPSS 25.0 (Chicago, IL) was used for all statistical analyses. Results are expressed as mean and standard deviation. Data were checked for normality using the Shapiro–Wilk test and log-transformed when non-normality was detected. A repeated measures analysis of variance (ANOVA) was then performed for IL-6 and body temperature measures, followed by post-hoc pairwise comparisons in case of a statistically significant main effect. In cases where sphericity assumptions were violated, Greenhouse–Geisser corrections were applied. The effect size (ES; Cohen's d) was calculated for IL-6 outcomes using the difference between Pre- and Post-change scores in each condition (0.20–0.50 small, 0.50–0.80 moderate, > 0.80 large effect (Cohen 1992). Changes in perceptual responses and energy expenditure were analysed non-parametrically. First, the Friedman test was used to test for an effect of time in each condition, whereafter Wilcoxon signed-rank tests were performed to compare individual time points between conditions. Finally, correlations were computed using Spearman’s r, investigating bivariate relationships between temperature and thermal perception variables, as well as temperature and plasma markers measured at the end of the intervention. P values are reported without a Bonferroni correction (Perneger 1998). For all analyses, the statistical significance threshold was set at P < 0.05.
Results
Body temperature
A main effect of time (F(6,66) = 41.0, P < 0.001), condition (F(2,22) = 13.0, P < 0.001) and a time by condition interaction (F(12,132) = 41.0, P < 0.001) indicated a differential Trec increase between conditions. The peak Trec did not differ between DH (38.0 ± 0.4 °C) and HWI (37.9 ± 0.2 °C; F(2,22) = 45.9, P = 0.16; Fig. 2A) but was higher in both conditions compared with CON (37.0 ± 0.3 °C; F(2,22) = 45.9, P ≤ 0.004).
Acute changes in rectal temperature (A), mean skin temperature (B) and skin temperatures for arm (C), chest (D), thigh (E) and calf (F) in response to Thermoneutral temperature (CON), dry heat (DH) and hot water immersion (HWI). Data reported as mean and standard deviation. Significant difference between *DH and CON, ΧHWI and CON and ΔDH and HWI (P < 0.05), determined via ANOVA
A main effect of time (F(6,66) = 585.3, P < 0.001), condition (F(2,22) = 271.6, P < 0.001), and time by condition interaction (F(12,132) = 108.5, P < 0.001) was found for mean Tskin. Mean Tskin was higher in DH (38.5 ± 1.2 °C), compared with HWI (36.1 ± 0.6 °C); and was higher throughout both these conditions compared with CON (31.6 ± 0.7 °C; F(2,22) ≥ 208.8, P < 0.001, Fig. 2B). The rate of rise in mean Tskin during the first 15 min was 0.53 °C/min in DH and 0.35 °C/min in HWI.
Arm and thigh Tskin were higher for DH than for HWI across the entire intervention (F(2,22) ≥ 68.2, P ≤ 0.013, Fig. 2C, E). Chest and calf Tskin during DH and HWI were higher than during CON (F(2,22) ≥ 22.4, P < 0.001, Fig. 2D, F).
Inflammatory response
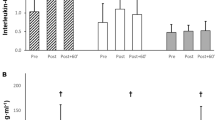
A main effect of time (F(2,22) = 28.4, P < 0.001), condition (F(2,22) = 4.1, P = 0.031), and time by condition interaction (F(4,44) = 6.3, P = 0.007) was found for plasma IL-6 concentration (Fig. 3). Immediately post heating, plasma concentrations of IL-6 were elevated in DH (F(2,22) = 7.1, P = 0.005) and HWI (F(2,22) = 7.1, P = 0.011) compared with CON; however, DH and HWI did not differ from each other (F(2,22) = 7.1, P = 0.458). At post 60 min, IL-6 concentrations were higher for DH than CON (F(2,22) = 4.4, P = 0.019), but HWI did not differ from CON (F(2,22) = 4.4, P = 0.067). There was no difference in plasma IL-6 concentrations between Pre and Post in CON (P = 0.943; d = 0.079), but plasma IL-6 concentrations significantly increased from pre to post in DH (P = 0.045; d = 0.925) and HWI (P = 0.003; d = 0.412). Plasma IL-6 concentrations were different between Pre and Post 60 min in all conditions; CON (P = 0.046; d = 0.228), DH (P = 0.011; d = 0.598) and HWI (P = 0.009; d = 0.456).
Perceptual responses
During both DH and HWI, basic affect, thermal sensation, thermal comfort, face wetness and body wetness changed over time (P ≤ 0.002), which was not the case for CON (P > 0.130, Table 1). From fifteen min into the session, basic affect scores were more unfavourable for DH when compared with CON (P ≤ 0.041), whilst HWI and CON did not differ at 15 min (P = 0.380) and at 45 min (P = 0.060) of the intervention. From 30 min, basic affect was more unfavourable during DH (P ≤ 0.007). During DH, basic affect was in the range of − 1 (“fairly bad”), whilst it was in the range of 1–2 (“fairly good” to “good”) during HWI and CON.
Thermal sensation differed between all conditions at all time points of the intervention; the most unfavourable scores were reported during DH, followed by HWI and CON (P ≤ 0.024, Table 1). Thermal comfort scores were most unfavourable for DH when compared with CON (P = 0.004), whilst HWI and CON did not differ at 15 min (P = 0.157). After 30 min, thermal comfort differed between all conditions (P ≤ 0.035) and was in the range of + 2 (“uncomfortable”) for DH; in the range of + 1 (“slightly uncomfortable”) for HWI and in the range of 0 (“comfortable”) for CON. None of the perceptual responses differed in the recovery period (P > 0.10 for all comparisons).
Perceived face wetness for DH and HWI were both higher than for CON at all time points of the intervention (P ≤ 0.011); DH face wetness was greater at 30 and 60 min than HWI (P ≤ 0.025). Perceived body wetness for DH was higher than HWI at baseline (P = 0.023). During DH and HWI, body wetness scores were greater throughout the intervention period, including 30 min after the intervention than CON, and remained higher for HWI after 60 min (P ≤ 0.036).
Enjoyment scores were lower for DH (8.7 ± 5.2) than for HWI (13.5 ± 2.9; P = 0.013) and CON (12.6 ± 2.9; P = 0.021), with no difference between HWI and CON (P = 0.126). Fondness scores were higher for HWI (6.3 ± 1.8) than for CON (4.5 ± 2.6; P = 0.038). There was no difference in fondness for DH (4.7 ± 2.3) compared with CON (P = 0.905) and HWI (P = 0.097).
HWI was selected as the favourite condition by N = 7, DH by N = 3, and CON by N = 2. Qualitative feedback following the intervention to support this choice included: “DH is slightly uncomfortable”; “DH is intensely hot”; “DH is easier than HWI”; “More pleasant/relaxing in the water”; “HWI more comfortable than DH”; “Not too hot on the skin in HWI”.
Cardiovascular and sweat response
A main effect of time (P < 0.001), condition (P < 0.001) and a time by condition interaction (P < 0.001) indicated a differential HR increase between conditions. Heart rate was higher for DH and HWI when compared with CON during the experimental period, and following that, HR for HWI remained higher than CON at one hour post (P < 0.001).
Energy expenditure for DH and HWI changed over time (P ≤ 0.001), with values in DH higher than CON at 60 min (P = 0.028). Sweat loss was higher during DH and HWI when compared with CON (P ≤ 0.001). In addition, sweat loss was higher following DH compared to HWI (P < 0.001, Table 2).
Bivariate relationships
In DH, thermal sensation correlated with arm Tskin (r = − 0.61, P = 0.034), and facial wetness was significantly correlated with body wetness (r = 0.65, P = 0.023). In HWI, basic affect was correlated with mean Tskin (r = 0.65, P = 0.024), and thermal sensation was correlated with calf Tskin (r = 0.71, P = 0.010). There was no relationship between face wetness and body wetness in HWI (r = 0.41, P = 0.190). In addition, IL-6 plasma concentrations did not correlate significantly with Trec (DH: P = 0.505, HWI: P = 0.649, CON: P = 0.412) and mean Tskin (DH: P = 0.991, HWI: P = 0.457, CON: P = 0.233) in any of the conditions.
Discussion
This study investigated the acute effects of Trec-matched DH and HWI on inflammatory as well as perceptual responses. The main results were: (1) mean Tskin during DH was higher when compared with HWI; (2) the plasma IL-6 response did not differ between DH and HWI; (3) basic affect, thermal comfort, and thermal sensation during HWI were more positive when compared with DH.
Inflammatory response
As DH and HWI showed the same IL-6 and Trec increase but differed substantially in the Tskin increase, the results of the present study may therefore imply that Trec is a more important driver to elevate IL-6 plasma concentration than Tskin . The Trec increase in the present study was relatively modest (~ 0.7 °C), which explains the relatively modest increase in IL-6 plasma concentration (~ twofold). Previous passive heating studies employed more aggressive heating protocols (Trec increase ~ 1–2 °C) and observed ~ two- to threefold increases in IL-6 plasma concentrations (Leicht et al. 2015; Faulkner et al. 2017; Hoekstra et al. 2018). This is in line with the suggested dose–response relationship between heat exposure, associated elevated Trec and the acute IL-6 response (Brunt et al. 2018; Hoekstra et al. 2020).
In the present study, the measure for peripheral temperature, mean Tskin, was higher in DH than in HWI. Tskin has been suggested to be related to sympathetic nervous system activity (Andrasik and Rime 2011), as the latter plays an important role in thermoregulation by regulating skin blood flow and perspiration (Collins 2013). As sympathetic activation is associated with the IL-6 response (Okamoto et al. 2015), the higher Tskin during DH may have been expected to augment the IL-6 response via greater sympathetic activation. However, HR, another indicator of sympathetic activity, was not different between DH and HWI, suggesting that sympathetic activation may have been similar between these trials.
In addition to sympathetic activation, the acute heat stress-induced inflammatory response may be influenced by peripheral tissue temperature (Kaldur et al. 2016). For instance, an ex vivo study showed increased IL-6 mRNA and protein expression during skeletal muscle heating (Welc et al. 2012). Further, Hoekstra et al., (2021) showed that the IL-6 response during passive heating was not blunted when the Trec increase during heating of the legs was blunted by upper body cooling. Therefore, it may be that a large increase in Trec is not required for a whole-body IL-6 response to occur as long as skeletal muscle, a main producer of IL-6 (Welc et al. 2012), is heated. Despite this reported influence of peripheral (muscle and/or skin) temperature on the IL-6 response, DH, which led to a higher rise in Tskin, did not lead to higher IL-6 concentrations in the current study. Here we must note that it is also possible that the observed differences in Tskin did not translate to divergent muscle temperatures (not directly measured in this study) between DH and HWI. A previous DH study showed that thigh Tskin was higher than the lateral femoral muscle temperature (Raccuglia et al. 2016). Conversely, a HWI study using 42 °C water reported higher lateral femoral muscle temperatures than Tskin (Rodrigues et al. 2020). Thus, this discrepancy between muscle temperature and Tskin for different modes of heating does not allow firm predictions of muscle temperature in the present study, which would require direct measures of muscle temperature in follow-up studies.
Elevated basal concentrations of IL-6 have repeatedly been associated with vascular dysfunction and impaired metabolic health (de Winther et al. 2005). Repeated passive heating can exert a downregulating effect on basal IL-6 concentration. For example, a study involving two weeks of hot spring bathing revealed a reduction in circulating IL-6 concentration among patients diagnosed with chronic heart failure (Oyama et al. 2013), highlighting the potential therapeutic impact of heat stress on modulating inflammatory responses. The current study indicates that even modest heat stress can induce an acute elevation in IL-6 concentration. This is in line with other studies, as heating of peripheral tissue resulting in a moderate rise in Trec (~ 0.6 °C) has demonstrated the potential to acutely elevate plasma IL-6 levels (Kaldur et al. 2016). Importantly, the comparable increase in IL-6 concentration observed following HWI and DH implies that individuals may make their selection of heating modality based on their personal preference or practical considerations.
Perceptual responses
It has been suggested that thermal sensation is mainly affected by Tskin, while thermal comfort is affected by both Tskin and Trec (Kato et al. 2001). In the present study, Tskin during DH was higher than during HWI, whilst the increase in Trec was similar between these conditions. In addition, the thermal sensation was greater in DH compared to HWI, and thermal comfort was more negative in DH than in HWI after 30 min of passive heating, indicating that DH was less tolerable than HWI. Our data hence support the mechanistic role of Tskin in thermal perceptions (Chatonnet and Cabanac 1965) and support the suggestion that the higher Tskin in DH may be the reason why DH is perceived as hotter and more uncomfortable than Trec-matched HWI. This is supported by correlational analyses from the current study, where Tskin outcomes were associated with thermal sensation and basic affect in both DH and HWI, respectively. Our findings are further corroborated by a previous study documenting a majority of participants not being able to complete 60 min of sauna exposure (55 °C, 54% relative humidity) due to discomfort, whereas all tolerated 60 min of water immersion (40 °C) resulting in similar Trec, but lower Tskin when compared with sauna exposure (Campbell et al. 2022). Of note, while Tskin during DH and HWI most likely reflects the exposure of the skin to the different thermal stimulus (i.e., water of ~ 37.5 °C and air of ~ 70 °C), it is important to acknowledge the potential direct impact of the water and air temperatures on the Tskin readings as a limitation.
Face wetness and body wetness perception were affected differently by DH and HWI. Face wetness perception was more pronounced in DH than in HWI, which may be related to the higher sweat rate during DH. Importantly, the higher face wetness during DH may also have affected the thermal comfort in this condition, as a correlation between these variables has been identified previously (Fukazawa and Havenith 2009). In DH, although dry air allows the body to dissipate heat through sweat evaporation, it must be noted that the Cocoon POD is a closed, capsule-like construction, limiting air flow to the skin and, therefore, evaporative heat loss. This may impair the evaporation efficiency of sweat, which increases the sensation of heat (Lee et al. 2011).
HWI was rated highest in both enjoyment and fondness, as well as being most often selected favourite condition. As indicated by the Hedonic theory, behaviours are more likely to be repeated if they are associated with pleasure (Ekkekakis et al. 2008). Taking this into account, our findings suggest that HWI could potentially be a superior method to DH when prescribing long-term heat therapy. However, it is important to acknowledge the relatively small sample size in this study and the fact that HWI was chosen as favourite by 58% of participants rather than close to anonymously. Further research with a larger and more diverse group of participants would be beneficial to gain a more comprehensive understanding of the preferences and experiences related to these conditions.
Cardiovascular and sweat response
Heart rate was increased during both DH and HWI to ~ 90 beats per minute, which is common during sauna therapy, even though a maximum HR of up to 120–150 beats per minute has also been reported (Laukkanen et al. 2018). A difference between DH and HWI is the hydrostatic pressure exerted on the body (Arborelius et al. 1972). The hydrostatic pressure during HWI did not appear to affect HR in this study, consistent with a study conducted post-exercise with DH or HWI, that reported no difference in HR between the two heating modes (Ashworth et al. 2023). A lower HR during HWI might have been expected, as HWI can cause central blood volume expansion, increasing stroke volume whilst decreasing HR compared with non-immersion (Wilcock et al. 2006). It therefore appears that the effect of the heat stress on HR was more dominant than the effect of hydrostatic pressure. Finally, a previous study shows Tskin to be an important determinant of the sweat response when Trec is fixed (Gagnon and Crandall 2018). This aligns with our findings, where Tskin and sweat loss were greater for DH than for HWI while matched for the rise in Trec.
Conclusion
This study showed that when matched for the rise in Trec, DH and HWI result in a similar IL-6 response. At the same time, perceptual responses were more positive during HWI when compared with DH, which may be related to the lower mean Tskin during HWI. Reducing Tskin while ensuring an increase in Trec may thus enhance the tolerability of passive heating without impacting the IL-6 response.
Data Availability
Data will be made available upon request.
Abbreviations
- ANOVA:
-
Analysis of variance
- CON:
-
Control condition, thermoneutral dry exposure
- DH:
-
Dry heat
- HR:
-
Heart rate
- HWI:
-
Hot water immersion
- IL-6:
-
Interleukin-6
- Trec :
-
Rectal temperature
- Tskin :
-
Skin temperature
References
An J, Lee I, Yi Y (2019) The thermal effects of water immersion on health outcomes: an integrative review. Int J Environ Res Public Health 16:1280. https://doi.org/10.3390/ijerph16071280
Andrasik F, Rime C (2011) Biofeedback. In: Waldman, SD (ed) Pain management, 2nd edn. Elsevier, pp 954–962
Arborelius M, Ballidin UI, Lilja B, Lundgren CE (1972) Hemodynamic changes in man during immersion with the head above water. Aerosp Med 43:592–598. https://doi.org/10.2490/jjrm1963.42.138
Ashworth E, Cotter J, Kilding A (2023) Post-exercise, passive heat acclimation with sauna or hot-water immersion provide comparable adaptations to performance in the heat in a military context. Ergonomics 66:49–60. https://doi.org/10.1080/00140139.2022.2058096
Brunt VE, Minson CT (2021) Heat therapy: mechanistic underpinnings and applications to cardiovascular health. J Appl Physiol 130:1684–1704. https://doi.org/10.1152/japplphysiol.00141.2020
Brunt VE, Wiedenfeld-Needham K, Comrada LN, Minson CT (2018) Passive heat therapy protects against endothelial cell hypoxia-reoxygenation via effects of elevations in temperature and circulating factors. J Physiol 596:4831–4845. https://doi.org/10.1113/JP276559
Campbell HA, Akerman AP, Kissling LS et al (2022) Acute physiological and psychophysical responses to different modes of heat stress. Exp Physiol 107:429–440. https://doi.org/10.1113/EP089992
Chatonnet J, Cabanac M (1965) The perception of thermal comfort. Int J Biometeorol 9:183–193. https://doi.org/10.1007/BF02188475
Cheung SS, Sleivert GG (2004) Lowering of skin temperature decreases isokinetic maximal force production independent of core temperature. Eur J Appl Physiol 91:723–728. https://doi.org/10.1007/s00421-004-1062-0
Chiesa ST, Trangmar SJ, Kalsi KK et al (2015) Local temperature-sensitive mechanisms are important mediators of limb tissue hyperemia in the heat-stressed human at rest and during small muscle mass exercise. Am J Physiol Circ Physiol 309:H369–H380. https://doi.org/10.1152/ajpheart.00078.2015
Cohen J (1992) A power primer. Psychol Bull 112:155–159. https://doi.org/10.1037/0033-2909.112.1.155
Collins KJ (2013) Temperature regulation and the autonomic nervous system. In: Mathias CJ, Bannister R (eds) Autonomic failure, 5th edn. Oxford University Press, Oxford, pp 247–255
de Weir JBV (1949) New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 109:1–9. https://doi.org/10.1113/jphysiol.1949.sp004363
de Winther MPJ, Kanters E, Kraal G, Hofker MH (2005) Nuclear factor κB Signaling in atherogenesis. Arterioscler Thromb Vasc Biol 25:904–914. https://doi.org/10.1161/01.ATV.0000160340.72641.87
Dill DB, Costill DL (1974) Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol 37:247–248. https://doi.org/10.1152/jappl.1974.37.2.247
Ekkekakis P, Hall EE, Petruzzello SJ (2008) The Relationship between exercise intensity and affective responses demystified: to crack the 40-year-old nut, replace the 40-year-old nutcracker! Ann Behav Med 35:136–149. https://doi.org/10.1007/S12160-008-9025-Z
Ely BR, Clayton ZS, McCurdy CE et al (2017) Meta-inflammation and cardiometabolic disease in obesity: can heat therapy help? Temp (austin, Tex) 5:9–21. https://doi.org/10.1080/23328940.2017.1384089
Ely BR, Francisco MA, Halliwill JR et al (2019) Heat therapy reduces sympathetic activity and improves cardiovascular risk profile in women who are obese with polycystic ovary syndrome. Am J Physiol Integr Comp Physiol 317:R630–R640. https://doi.org/10.1152/ajpregu.00078.2019
Epstein Y, Moran DS (2006) Thermal comfort and the heat stress indices. Ind Health 44:388–398. https://doi.org/10.2486/indhealth.44.388
Faulkner SH, Jackson S, Fatania G, Leicht CA (2017) The effect of passive heating on heat shock protein 70 and interleukin-6: a possible treatment tool for metabolic diseases? Temperature 4:292–304. https://doi.org/10.1080/23328940.2017.1288688
Filingeri D, Fournet D, Hodder S, Havenith G (2015) Tactile cues significantly modulate the perception of sweat-induced skin wetness independently of the level of physical skin wetness. J Neurophysiol 113:3462–3473. https://doi.org/10.1152/JN.00141.2015
Frank SM, Raja SN, Bulcao CF, Goldstein DS (1999) Relative contribution of core and cutaneous temperatures to thermal comfort and autonomic responses in humans. J Appl Physiol 86:1588–1593. https://doi.org/10.1152/jappl.1999.86.5.1588
Fukazawa T, Havenith G (2009) Differences in comfort perception in relation to local and whole body skin wettedness. Eur J Appl Physiol 106:15–24. https://doi.org/10.1007/s00421-009-0983-z
Gagge AP, Nishi Y (1976) Physical indices of the thermal environment. ASHRAE J 18:47–51
Gagnon D, Crandall CG (2018) Sweating as a heat loss thermoeffector. Handb Clin Neurol 156:211–232. https://doi.org/10.1016/B978-0-444-63912-7.00013-8
Goto K, Oda H, Kondo H et al (2011) Responses of muscle mass, strength and gene transcripts to long-term heat stress in healthy human subjects. Eur J Appl Physiol 111:17–27. https://doi.org/10.1007/s00421-010-1617-1
Hardy CJ, Rejeski WJ (1989) Not what, but how one feels: the measurement of affect during exercise. J Sport Exerc Psychol 11:304–317. https://doi.org/10.1123/jsep.11.3.304
Hashizaki T, Nishimura Y, Teramura K et al (2018) Differences in serum IL-6 response after 1 °C rise in core body temperature in individuals with spinal cord injury and cervical spinal cord injury during local heat stress. Int J Hyperth 35:541–547. https://doi.org/10.1080/02656736.2018.1511838
Helamaa E, Aikas E (1988) The secret of good ‘löyly.’ Ann Clin Res 20:224–229
Hoekstra SP, Bishop NC, Faulkner SH et al (2018) Acute and chronic effects of hot water immersion on inflammation and metabolism in sedentary, overweight adults. J Appl Physiol 125:2008–2018. https://doi.org/10.1152/japplphysiol.00407.2018
Hoekstra SP, Bishop NC, Leicht CA (2020) Elevating body temperature to reduce low-grade inflammation: a welcome strategy for those unable to exercise? Exerc Immunol Rev 26:42–55
Hoekstra SP, Ogawa T, Dos Santos M et al (2021) The effects of local versus systemic passive heating on the acute inflammatory, vascular and glycaemic response. Appl Physiol Nutr Metab 46:808–818. https://doi.org/10.1139/apnm-2020-0704
Jezova D, Radikova Z, Vigas M (2007) Growth hormone response to different consecutive stress stimuli in healthy men: Is there any difference? Stress 10:205–211. https://doi.org/10.1080/10253890701292168
Jiang R, Wang Y (2020) Study of the human stickiness perception of wet fabric on the volar forearm via two contact modes: friction and adhesion-separation. Perception 49:1311–1332. https://doi.org/10.1177/0301006620976992
Jung ME, Bourne JE, Little JP (2014) Where does HIT fit? An examination of the affective response to high-intensity intervals in comparison to continuous moderate- and continuous vigorous-intensity exercise in the exercise intensity-affect continuum. PLoS One 9:e114541. https://doi.org/10.1371/journal.pone.0114541
Kaldur T, Unt E, Ööpik V et al (2016) The acute effects of passive heat exposure on arterial stiffness, oxidative stress, and inflammation. Medicina (b Aires) 52:211–216. https://doi.org/10.1016/j.medici.2016.06.001
Kato M, Sugenoya J, Matsumoto T et al (2001) (2001) The effects of facial fanning on thermal comfort sensation during hyperthermia. Pflügers Arch 4432(443):175–179. https://doi.org/10.1007/S004240100681
Kikuchi H, Shiozawa N, Takata S et al (2013) Effect of repeated Waon therapy on exercise tolerance and pulmonary function in patients with chronic obstructive pulmonary disease: a pilot controlled clinical trial. Int J Chron Obstr Pulm Dis 9:9. https://doi.org/10.2147/COPD.S50860
Kukkonen-Harjula K, Oja P, Laustiola K et al (1989) Haemodynamic and hormonal responses to heat exposure in a Finnish sauna bath. Eur J Appl Physiol Occup Physiol 58:543–550. https://doi.org/10.1007/BF02330710
Kunutsor SK, Khan H, Laukkanen T, Laukkanen JA (2018) Joint associations of sauna bathing and cardiorespiratory fitness on cardiovascular and all-cause mortality risk: a long-term prospective cohort study. Ann Med 50:139–146. https://doi.org/10.1080/07853890.2017.1387927
Laing SJ, Jackson AR, Walters R et al (2008) Human blood neutrophil responses to prolonged exercise with and without a thermal clamp. J Appl Physiol 104:20–26. https://doi.org/10.1152/JAPPLPHYSIOL.00792.2007
Laukkanen T, Khan H, Zaccardi F, Laukkanen JA (2015) Association between sauna bathing and fatal cardiovascular and all-cause mortality events. JAMA Intern Med 175:542–548. https://doi.org/10.1001/jamainternmed.2014.8187
Laukkanen JA, Laukkanen T, Kunutsor SK (2018) Cardiovascular and other health benefits of sauna bathing: a review of the evidence. Mayo Clin Proc 93:1111–1121. https://doi.org/10.1016/j.mayocp.2018.04.008
Lee J-Y, Nakao K, Tochihara Y (2011) Validity of perceived skin wettedness mapping to evaluate heat strain. Eur J Appl Physiol 111:2581–2591. https://doi.org/10.1007/s00421-011-1882-7
Leicht CA, Kouda K, Umemoto Y et al (2015) Hot water immersion induces an acute cytokine response in cervical spinal cord injury. Eur J Appl Physiol 115:2243–2252. https://doi.org/10.1007/S00421-015-3206-9
Mansfield RG, Hoekstra SP, Bill JJ, Leicht CA (2021) Local cooling during hot water immersion improves perceptions without inhibiting the acute interleukin-6 response. Eur J Appl Physiol 121:1581–1591. https://doi.org/10.1007/S00421-021-04616-5
Martin B, Ji S, White CM et al (2010) Dietary energy intake, hormesis, and health. Hormesis A Revolut Biol Toxicol Med. https://doi.org/10.1007/978-1-60761-495-1_7
McCarty MF, Barroso-Aranda J, Contreras F (2009) Regular thermal therapy may promote insulin sensitivity while boosting expression of endothelial nitric oxide synthase—effects comparable to those of exercise training. Med Hypotheses 73:103–105. https://doi.org/10.1016/j.mehy.2008.12.020
Nadel EH (1977) Thermal and energetic exchanges during swimming. Probl with Temp Regul Dur Exerc 20:91–119
Okamoto LE, Raj SR, Gamboa A et al (2015) Sympathetic activation is associated with increased IL-6, but not CRP in the absence of obesity: lessons from postural tachycardia syndrome and obesity. Am J Physiol Circ Physiol 309:H2098–H2107. https://doi.org/10.1152/ajpheart.00409.2015
Oyama JI, Kudo Y, Maeda T et al (2013) Hyperthermia by bathing in a hot spring improves cardiovascular functions and reduces the production of inflammatory cytokines in patients with chronic heart failure. Heart Vessels 28:173–178. https://doi.org/10.1007/S00380-011-0220-7
Perneger TV (1998) What’s wrong with Bonferroni adjustments. BMJ Br Med J 316:1236. https://doi.org/10.1136/BMJ.316.7139.1236
Petersen AMW, Pedersen BK (2005) The anti-inflammatory effect of exercise. J Appl Physiol 98:1154–1162. https://doi.org/10.1152/japplphysiol.00164.2004
Raccuglia M, Lloyd A, Filingeri D et al (2016) Post-warm-up muscle temperature maintenance: blood flow contribution and external heating optimisation. Eur J Appl Physiol 116:395. https://doi.org/10.1007/S00421-015-3294-6
Ramanathan NL (1964) A new weighting system for mean surface temperature of the human body. J Appl Physiol 19:531–533. https://doi.org/10.1152/jappl.1964.19.3.531
Rodrigues P, Trajano GS, Wharton L, Minett GM (2020) Muscle temperature kinetics and thermoregulatory responses to 42 °C hot-water immersion in healthy males and females. Eur J Appl Physiol 120:2611–2624. https://doi.org/10.1007/S00421-020-04482-7
Simkin P, Bolding A (2004) Update on nonpharmacologic approaches to relieve labor pain and prevent suffering. J Midwifery Womens Health 49:489–504. https://doi.org/10.1016/J.JMWH.2004.07.007
Simmons EE, Bergeron ER, Florian JP (2017) The impact of repetitive long-duration water immersion on vascular function. PLoS One 12:e0181673. https://doi.org/10.1371/journal.pone.0181673
Steensberg A, Fischer CP, Keller C, et al (2003) IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol Endocrinol Metab 285:E433–7. https://doi.org/10.1152/ajpendo.00074.2003
Welc SS, Phillips NA, Oca-Cossio J et al (2012) Hyperthermia increases interleukin-6 in mouse skeletal muscle. Am J Physiol Physiol 303:C455–C466. https://doi.org/10.1152/ajpcell.00028.2012
Wilcock IM, Cronin JB, Hing WA (2006) Physiological response to water immersion: a method for sport recovery? Sports Med 36:747–765. https://doi.org/10.2165/00007256-200636090-00003
Yildirim N, Filiz Ulusoy M, Bodur H (2010) The effect of heat application on pain, stiffness, physical function and quality of life in patients with knee osteoarthritis. J Clin Nurs 19:1113–1120. https://doi.org/10.1111/j.1365-2702.2009.03070.x
Zaccardi F, Laukkanen T, Willeit P et al (2017) Sauna bathing and incident hypertension: a prospective cohort study. Am J Hypertens 30:1120–1125. https://doi.org/10.1093/ajh/hpx102
Acknowledgements
This research was supported by the Peter Harrison Centre for Disability Sport. Yunuo Su was supported by the China Scholarship Council. The authors would thank Bin Ngiam, Grace Carson and Holly Lloyd-Jones for assistance during data collection.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by YS and SH. All authors were responsible for data interpretation and revision. The first draft of the manuscript was written by YS, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants adhered to the standards of the 1964 Helsinki Declaration and its later amendments or comparable ethical standards and were approved by the institutional ethics committee.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Communicated by Fabio fischetti.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Su, Y., Hoekstra, S.P. & Leicht, C.A. Hot water immersion is associated with higher thermal comfort than dry passive heating for a similar rise in rectal temperature and plasma interleukin-6 concentration. Eur J Appl Physiol 124, 1109–1119 (2024). https://doi.org/10.1007/s00421-023-05336-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-023-05336-8