Abstract
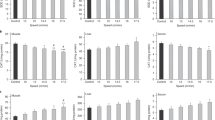
The present study investigated mitochondrial adaptations and oxidative damage after 4 and 8 weeks of running training in skeletal muscle of mice. Twenty-one male mice (CF1, 30–35 g) were distributed into the following groups (n = 7): untrained (UT); trained—4 weeks (T4); trained—8 weeks (T8). Forty-eight hours after the last training session the animals were killed by decapitation and quadriceps (red portion) were removed and stored at −70°C. Succinate dehydrogenase (SDH), complexes I, II, II–III and IV, lipoperoxidation (TBARS), protein carbonyls (PC) and total thiol content were measured. Results show that endurance training (8-wk) increases the SDH activity and complexes (I, II, III, IV), decreases oxidative damage (TBARS, CP) and increases total thiol content in skeletal muscle when compared to untrained animals. In conclusion, eight weeks of running training are necessary for increases in mitochondrial respiratory chain enzyme activities to occur, in association with decreased oxidative damage.




Similar content being viewed by others
References
Aguiar AS Jr, Talita T, Cleber AP, Luciano AS, Ana CA, Flávio K, João Q, Emílio LS, Ricardo AP (2008) Intense exercise induces mitochondrial dysfunction in mice brain. Neurochem 33:51–58
Aguiló A, Tauler P, Fuentespina E, Tur JA, Córdova A, Pons A (2005) Antioxidant response to oxidative stress induced by intense exercise. Physiol Behav 84:1–7
Aksenov MY, Markesberya WR (2001) Changes in thiol content and expression of glutathione redox system genes in the hippocampus and cerebellum in Alzheimer’s disease. Neurosci Lett 302:141–145
Anttila K, Mänttäri S, Järvilehto M (2006) Effects of different training protocols on Ca2+ handling and oxidative capacity in skeletal muscle of Atlantic salmon. Exp Biol 209:2971–2978
Ascensão AA, Magalhães JF, Soares JM, Ferreira RM, Neuparth MJ, Appell HJ, Duarte JA (2005) Cardiac mitochondrial respiratory function and oxidative stress: the role of exercise. Int J Sports Med 26:258–267
Astrand PO, Hultman E, Juhlin DA, Reynolds G (1986) Disposal of lactate during and after strenuous exercise in humans. J Appl Physiol 61:338–343
Baeuerle PA, Baltimore D (1998) Activation of DNA binding activity in an apparently cytoplasmic precursor of the NF-κB transcriptional factor. Cell 53:211–217
Brechue WF, Pollock ML (1996) Exercise training for coronary artery disease in the elderly. Clin Geriatr Med 12:207–229
Boveris A, Chance B (1973) The mitochondrial production of hydrogen peroxide: general properties and the effect of hyperbaric oxygen. Biochem J 134:707–716
Calabrese V, Lodi R, Tonon C, D’Agata V, Sapienza M, Scapagnini G, Mangiameli A, Pennisi G, Stella AM, Butterfield DA (2005) Oxidative stress, mitochondrial dysfunction and cellular stress response in Friedreich’s ataxia. J Neurol Sci 233:145–162
Cassina A, Radi R (1996) Differential inhibitory Aation of nitric oxide and peroxynitrite on mitochondrial electron transport. Arch Biochem Biophys 328:309–316
Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, Kramer AF (2003) Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol Ser A Biol Sci Med Sci 58:176–180
Delp MD, Duan C (1996) Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J Appl Physiol 80:261–270
Draper HH, Hadley M (1990) Malondialdehyde determination as index of lipid peroxidation. Meth Enzymol 186:421–431
Ecochard L, Lhenry F, Sempore B, Favier R (2000) Skeletal muscle HSP72 level during endurance training: influence of peripheral arterial insufficiency. Pflugers Arch 440:918–924
Fischer JC, Ruitenbeek W, Berden JA, Trijbels JM, Veerkamp JH, Stadhouders AM, Sengers RC, Janssen AJ (1985) Differential investigation of the capacity of succinate oxidation in human skeletal muscle. Clin Chim Acta 153:23–26
Halliwell B, Gutteridge JM (2007) Free radical in biology and medicine. Oxford University Press, Oxford
Hessel E, Haberland A, Müller M, Lerche D, Schimke I (2000) Oxygen radical generation of neutrophils: a reason for oxidative stress during marathon running? Clin Chim Acta 2:145–156
Hollander J, Fiebig R, Gore M, Ookawara T, Ohno H, Ji LL (2001) Superoxide dismutase gene expression is activated by a single bout of exercise. Pflug Arch 442:426–434
Holloszy JO, Smith EK, Vining M, Adams S (1985) Effect of voluntary exercise on longevity of rats. J Appl Physiol 59:826–831
Itoh H, Ohkuwa T, Yamamoto T, Sato Y, Miyamura M, Naoi M (1998) Effects of endurance physical training on hydroxyl radical generation in rat tissues. Life Sci 63:1921–1929
Judge S, Jang YM, Smith A, Selman C, Phillips T, Speakman JR, Hagen T, Leeuwenburgh C (2005) Exercise by lifelong voluntary wheel running reduces subsarcolemmal and interfibrillar mitochondrial hydrogen peroxide production in the heart. Am J Physiol Regul Integr Comp Physiol 289:1564–1572
Keeney PM, Xie J, Capaldi RA, Bennett JP Jr (2006) Parkinson’s disease brain mitochondrial complex I has oxidatively damaged subunit and is functionally impaired and misassembled. J Neurosci 19:5256–5264
Lambertucci RH, Levada-Pires AC, Rossoni LV, Curi R, Pithon-Curi TC (2006) Effects of aerobic exercise training on antioxidant enzyme activities and mRNA levels in soleus muscle from young and aged rats. Mech Ageing Dev 128:267–275
Laughlin MH, Simpson T, Sexton WL, Brown OR, Smith JK, Korthuis RJ (1990) Skeletal muscle oxidative capacity, antioxidant enzymes, and exercise training. J Appl Physiol 68:2337–2343
Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Stadtman ER (1990) Determination of carbonyl content in oxidatively modified proteins. Meth Enzymol 186:464–478
Locke M (1997) The cellular stress response to exercise: role of stress proteins. Exerc Sport Sci Rev 25:105–136
Lowry OH, Rosebough NG, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Magalhães J, Ferreira R, Marques F, Olivera E, Soares J, Ascensão A (2007) Indoor climbing elicits plasma oxidative stress. Med Sci Sports Exerc 39:955–963
Mastaloudis A, Leonard SW, Traber MG (2001) Oxidative stress in athletes during extreme endurance exercise. Free Radic Biol Med 31:911–922
Menshikova EV, Ritov VB, Ferrell RE, Azuma K, Goodpaster BH, Kelley DE (2007) Characteristics of skeletal muscle mitochondrial biogenesis induced by moderate-intensity exercise and weight loss in obesity. J Appl Physiol 103:21–27
Navarro AA, Cañabate C, Sanchez PMJ (1999) Myocardial and skeletal muscle aging and changes in oxidative stress in relationship to rigorous exercise training. Mech Ageing 108:207–217
Navarro A, Gomez C, Lopez-Cepero JM, Boveris A (2004) Beneficial effects of moderate exercise on mice aging: survival, behavior, oxidative stress, and mitochondrial electron transfer. Am J Physiol Regul Integr Comp Physiol 286:505–511
Ogonovszky H, Sasvári M, Dosek A, Berkes I, Kaneko T, Tahara S, Nakamoto H, Goto S, Radák Z (2005) The effects of moderate, strenuous, and overtraining on oxidative stress markers and DNA repair in rat liver. Can J Appl Physiol 30:186–195
Oh-Ishi S, Kizaki T, Nagasawa J, Izawa T, Komabayashi T, Nagata N, Suzuki K, Taniguchi N, Ohno H (1997) Effects of endurance training on superoxide dismutase activity, content and mRNA expression in rat muscle. Clin Exp Pharmacol Physiol 24:326–332
Olert ED, Cross BM, Mcwillians AA (1993) Guide to care and use of experimental animals canadian council on animal. Ottawa
Pinho RA, Andrades ME, Oliveira MR, Pirola AC, Zago MS, Silveira PC, Dal-Pizzol Moreira FJC (2006) Imbalance in SOD/CAT activities in rat skeletal muscles submitted to treadmill training exercise. Cell Biol Int 30:848–853
Powers SK, Locke M, Demirel HA (2001) Exercise, heat shock proteins, and myocardial protection from I-R injury. Med Sci Sports Exerc 33:386–392
Powers SK, Criswell D, Lawler J, Ji LL, Martin D, Herb RA, Dudley G (1994) Influence of exercise and fiber type on antioxidant enzyme activity in rat skeletal muscle. Am J Physiol 266:375–380
Radak Z, Chung HY, Goto S (2005) Exercise and hormesis: oxidative stress-related adaptation for successful aging. Biogerontology 6:71–75
Radak Z, Naito H, Kaneko T, Tahara S, Nakamoto H, Takahashi R, Cardozo-Pelaez F, Goto S (2002) Exercise training decreases DNA damage and increases DNA repair and resistance against oxidative stress of proteins in aged rat skeletal muscle. Pflugers Arch 445:273–278
Radak Z, Taylor AW, Ohno H, Goto S (2001) Adaptation to exerciseinduced oxidative stress: from muscle to brain. Exerc Immunol Rev 7:90–107
Rustin P, Chretien D, Bourgeron T, Gerard B, Rotig A, Saudubray JM, Munnich A (1994) Biochemical and molecular investigations in respiratory chain deficiencies. Clinica Chimica Acta 228:35–51
Saxton JM, Donnelly AE, Roper HP (1994) Indices of free-radical-mediated damage following maximum voluntary eccentric and concentric muscular work. Eur J Appl Physiol Occup Physiol 68:189–193
Senturk UK, Gunduz F, Kuru O, Aktekin MR, Kipmen D, Yalcin O, Bor-Kucukatay M, Yesilkaya A, Baskurt OK (2001) Exercise-induced oxidative stress affects erythrocytes in sedentary rats but not exercise-trained rats. J Appl Physiol 91:1999–2004
Servais S, Couturier K, Koubi H, Rouanet JL, Desplanches D, Sornay-Mayet MH, Sempore B, Lavoie JM, Favier Roland (2003) Effect of voluntary exercise on H2O2 release by subsarcolemmal and intermyofibrillar mitochondria. Free Radic Biol Med 35:24–32
Siu PM, Donley DA, Bryner RW, Always SE (2003) Citrate Synthase expression and enzyme activity after endurance training in cardiac and skeletal muscles. J Appl Physiol 94:555–560
Stadhouders AM, Sengers RC, Janssen AJ (1985) Differential investigation of the capacity of succinate oxidation in human skeletal muscle. Clinica Chimica Acta 153:23–26
Starnes JW, Barnes BD, Olsen ME (2007) Exercise training decreases rat heart mitochondria free radical generation but does not prevent Ca2- induced dysfunction. J Appl Physiol 102:1793–1798
Sureda A, Tauler P, Aguiló A, Cases N, Fuentespina E, Córdova A, Tur JA, Pons A (2005) Relation between oxidative stress markers and antioxidant endogenous defences during exhaustive exercise. Free Radic Res 12:1317–1324
Szweda PA, Friguet B, Szweda LI (2002) Proteolysis, free radicals, and aging. Free Radic Biol Med 33:29–36
Tsai K, Hsu TG, Hsu KM, Cheng H, Liu TY, Hsu CF, Kong CW (2001) Oxidative DNA damage in human peripheral leukocytes induced by massive aerobic exercise. Free Radic Biol Med 11:1465–1472
Venditti P, Masullo P, Di Meo S (1999) Effect of training on H2O2 release by mitochondria from rat skeletal muscle. Arch Biochem Biophys 372:315–320
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Silva, L.A., Pinho, C.A., Scarabelot, K.S. et al. Physical exercise increases mitochondrial function and reduces oxidative damage in skeletal muscle. Eur J Appl Physiol 105, 861–867 (2009). https://doi.org/10.1007/s00421-008-0971-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-008-0971-8