Abstract
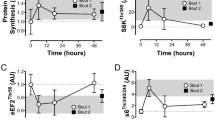
The purpose of this investigation was to determine if p70s6k phosphorylation is dependent on the mode of resistance exercise (e.g. isometric vs. lengthening). Two groups (n = 5 each) of Female Sprague Dawley rats, ∼12 weeks old, were tested. Rats were anesthetized and indwelling electrodes used to stimulate the right hind limb muscles via the sciatic nerve. The tibialis anterior (TA) muscle of Group 1 rats were exposed to three sets of ten isometric resistance contractions while the TA of Group 2 rats were exposed to three sets of ten resistance contractions that involved lengthening. Contralateral TA muscles served as non-exercised controls. The TA muscle was harvested 6 h post exercise and then the rat was euthanized. Muscle samples were processed to compare p70s6k phosphorylation between groups. A single bout of TA contractions that involved muscle lengthening resulted in significantly (p < 0.05) higher levels of phospho-p70s6k six hours post exercise compared to controls and isometric contractions. The differences in total p70s6k six hours post exercise were not significantly different between groups. Results suggest that signal transduction pathways activated by isometric exercise may differ (i.e., a non-p70s6k activation pathway) from that activated by lengthening exercise.




Similar content being viewed by others
References
Adams GR, Cheng DC, Haddad F, Baldwin KM (2004) Skeletal muscle hypertrophy in response to isometric, lengthening, and shortening training bouts of equivalent duration. J Appl Physiol 96(5):1613–1618
Baar K, Esser K (1999) Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol 276(1 Pt 1):C120–C127
Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD (2001) Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3(11):1014–1019
Bolster DR, Kimball SR, Jefferson LS (2003a) Translational control mechanisms modulate skeletal muscle gene expression during hypertrophy. Exerc Sport Sci Rev 31(3):111–116
Bolster DR, Kubica N, Crozier SJ, Williamson DL, Farrell PA, Kimball SR, Jefferson LS, (2003b) Immediate response of mammalian target of rapamycin (mTOR)-mediated signaling following acute resistance exercise in rat skeletal muscle. J Physiol 15;553(Pt 1):213–220
Bolster DR, Jefferson LS, Kimball SR (2004) Regulation of protein synthesis associated with skeletal muscle hypertrophy by insulin-, amino acid- and exercise-induced signaling. Proc Nutr Soc 63(2):351–356
Brozinick JT Jr, Birnbaum MJ (1998) Insulin, but not contraction, activates Akt/PKB in isolated rat skeletal muscle. J Biol Chem 12;273(24):14679–14682
Caiozzo VJ, Ma E, McCue SA, Smith E, Herrick RE, Baldwin KM (1992) A new animal model for modulating myosin isoform expression by altered mechanical activity. J Appl Physiol 73(4):1432–1440
Chesley A, MacDougall JD, Tarnopolsky MA, Atkinson SA, Smith K (1992) Changes in human muscle protein synthesis after resistance exercise. J Appl Physiol 73(4):1383–1388
Deldicque L, Theisen D, Francaux M (2005) Regulation of mTOR by amino acids and resistance exercise in skeletal muscle. Eur J Appl Physiol 94:1–10
Eliasson J, Elfegoun T, Nilsson J, Köhnke R, Ekblom B, Blomstrand E (2006) Maximal lengthening contractions increase p70S6 kinase phosphorylation in human skeletal muscle in the absence of nutritional supply. Am J Physiol Endocrinol Metab 291(6):E1197–E1205
Garfinkel S, Cafarelli E (1992) Relative changes in maximal force, EMG, and muscle cross-sectional area after isometric training. Med Sci Sports Exerc 24(11):1220–1227
Garma T, Kobayashi C, Haddad F, Adams GR, Bodell PW, Baldwin KM (2007) Similar acute molecular responses to equivalent volumes of isometric, lengthening, or shortening mode resistance exercise. J Appl Physiol 102(1):135–143
Hernandez JM, Fedele MJ, Farrell PA (2000) Time course evaluation of protein synthesis and glucose uptake after acute resistance exercise in rats. J Appl Physiol 88(3):1142–1149
Hornberger TA, Stuppard R, Conley KE, Fedele MJ, Fiorotto ML, Chin ER, Esser KA (2004) Mechanical stimuli regulate rapamycin-sensitive signalling by a phosphoinositide 3-kinase-, protein kinase B- and growth factor-independent mechanism. Biochem J 15;380(Pt 3):795–804
Hornberger TA, Chu WK, Mak YW, Hsiung JW, Huang SA, Chien S (2006) The role of phospholipase D and phosphatidic acid in the mechanical activation of mTOR signaling in skeletal muscle. Proc Natl Acad Sci USA 21;103(12):4741–4746
Ikai M, Fukunaga T (1970) A study on training effect on strength per unit cross-sectional area of muscle by means of ultrasonic measurement. Int Z Angew Physiol 28(3):173–180
Jefferies HBJ, Reinhard C, Kozma SC, Thomas G (1994a) Rapamycin selectively represses translation of the “polypyrimidine tract” mRNA family. Proc Natl Acad Sci USA 10;91(10):4441–4445
Jefferies HBJ, Thomas G, Thomas G (1994b) Elongation factor-1 alpha mRNA is selectively translated following mitogenic stimulation. J Biol Chem 11;269(6):4367–4372
Kanabara K, Sakai A, Watanabe M, Furuya E, Shimada M (1997) Distribution of fiber types determined by in situ hybridization of myosin heavy chain mRNA and enzyme histochemistry in rat skeletal muscles. Cell Mol Biol 43(3):319–327
Nader GA, Esser KA (2001) Intracellular signaling specificity in skeletal muscle in response to different modes of exercise. J Appl Physiol 90(5):1936–1942
Parkington JD, LeBrasseur NK, Siebert AP, Fielding RA (2004) Contraction-mediated mTOR, p70S6k, and ERK1/2 phosphorylation in aged skeletal muscle. J Appl Physiol 97(1):243–248
Rennie MJ, Wackerhage H, Spangenburg EE, Booth FW (2004) Control of the size of the human muscle mass. Annu Rev Physiol 66:799–828
Sakamoto K, Goodyear LJ (2002) Invited review: intracellular signaling in contracting skeletal muscle. J Appl Physiol 93(1):369–383
Sakamoto K, Hirshman MF, Aschenbach WG, Goodyear LJ (2002) Contraction regulation of Akt in rat skeletal muscle. J Biol Chem 277(14):11910–11917
Sakamoto K, Aschenbach WG, Hirshman MF, Goodyear LJ (2003) Akt signaling in skeletal muscle: regulation by exercise and passive stretch. Am J Physiol Endocrinol Metab 285(5):E1081–E1088
Sherwood DJ, Dufresne SD, Markuns JF, Cheatham B et al (1999) Differential regulation of MAP kinase, p70(S6K), and Akt by contraction and insulin in rat skeletal muscle. Am J Physiol 276(5 Pt 1):E870–E878
Spangenburg EE, McBride T (2006) Inhibition of stretch-activated channels during eccentric muscle contraction attenuates p70S6K activation. J Appl Physiol 100(1):129–135
Wong TS, Booth FW (1988) Skeletal muscle enlargement with weight-lifting exercise by rats. J Appl Physiol 65(2):950–954
Wong TS, Booth FW (1990a) Protein metabolism in rat gastrocnemius muscle after stimulated chronic concentric exercise. J Appl Physiol 69(5):1709–1717
Wong TS, Booth FW (1990b) Protein metabolism in rat tibialis anterior muscle after stimulated chronic eccentric exercise. J Appl Physiol 69(5):1718–1724
Acknowledgments
The authors wish to thank Emily Pettycrew for outstanding technical support. Also partial support to Dr E. E. Spangenburg was received from NIH (AR051396). Dr. E. E. Spangenburg current address is University of Maryland, Department of Kinesiology, College Park, MD 20742.
Conflict of Interest
The authors have no relationships that present a conflict of interest regarding the worked presented in this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Burry, M., Hawkins, D. & Spangenburg, E.E. Lengthening contractions differentially affect p70s6k phosphorylation compared to isometric contractions in rat skeletal muscle. Eur J Appl Physiol 100, 409–415 (2007). https://doi.org/10.1007/s00421-007-0444-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-007-0444-5