Abstract
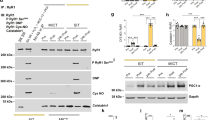
The ryanodine receptor type-I (RyR1) is one key player of the excitation–contraction coupling (E-CC) machinery. However, RyR1 expression in human skeletal muscle disuse and plasticity changes are not well documented. We studied the expression and the functional modifications of RyR1 following prolonged bed rest (BR) without and with exercise countermeasure (Resistive Vibration Exercise, RVE). Soleus biopsies were taken from a non-trained control (BR-CTRL) and trained (BR-RVE) group (each n = 10) before and after BR. In BR-CTRL group, a fibre type-specific immunopattern of RyR1 (type-I < type-II) was documented, and RyR1 immunofluorescence intensity and protein expression together with [3H]ryanodine binding were decreased after BR. In BR-RVE group, RyR1 immunosignals were increased and fiber type specificity was no longer present. RyR1 protein expression was unchanged, whereas [3H]ryanodine binding increased after BR. Confocal and biochemical analysis confirmed subcellular co-localisation and protein–protein interaction of RyR1 with nitric oxide (NO)-synthase type-1 (NOS1). S-nitrosylation of RyR1 was increased in BR-CTRLpost only, suggesting a reduction of RyR1 open channel probability by nitrosylation mechanisms following prolonged disuse. We conclude that following extended body deconditioning in bed rest, RVE countermeasure maintained normal RyR1 expression and nitrosylation patterns required for adequate E-CC in human performance control.







Similar content being viewed by others
References
Anttila K, Manttari S, Jarvilehto M (2006) Effects of different training protocols on Ca2+ handling and oxidative capacity in skeletal muscle of Atlantic salmon (Salmo salar L.). J Exp Biol 209:2971–2978
Aracena-Parks P, Goonasekera SA, Gilman CP, Dirksen RT, Hidalgo C, Hamilton SL (2006) Identification of cysteines involved in S-nitrosylation, S-glutathionylation, and oxidation to disulfides in ryanodine receptor type 1. J Biol Chem 281:40354–40368
Balon TW (1999) Integrative biology of nitric oxide and exercise. Exerc Sport Sci Rev 27:219–253
Barouch LA, Harrison RW, Skaf MW, Rosas GO, Cappola TP, Kobeissi ZA, Hobai IA, Lemmon CA, Burnett AL, O’Rourke B, Rodriguez ER, Huang PL, Lima JA, Berkowitz DE, Hare JM (2002) Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature 416:337–339
Bastide B, Conti A, Sorrentino V, Mounier Y (2000) Properties of ryanodine receptor in rat muscles submitted to unloaded conditions. Biochem Biophys Res Commun 270:442–447
Bergstrom J (1975) Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest 35:609–616
Berridge MJ (1993) Inositol trisphosphate and calcium signalling. Nature 361:315–325
Blottner D, Salanova M, Puttmann B, Schiffl G, Felsenberg D, Buehring B, Rittweger J (2006) Human skeletal muscle structure and function preserved by vibration muscle exercise following 55 days of bed rest. Eur J Appl Physiol 97:261–271
Brenman JE, Chao DS, Xia H, Aldape K, Bredt DS (1995) Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy. Cell 82:743–752
Buchwalow IB, Minin EA, Samoilova VE, Boecker W, Wellner M, Schmitz W, Neumann J, Punkt K (2005) Compartmentalization of NO signaling cascade in skeletal muscles. Biochem Biophys Res Commun 330:615–621
Caiozzo VJ, Haddad F, Baker MJ, Herrick RE, Prietto N, Baldwin KM (1996) Microgravity-induced transformations of myosin isoforms and contractile properties of skeletal muscle. J Appl Physiol 81:123–132
Chaudhari N (1992) A single nucleotide deletion in the skeletal muscle-specific calcium channel transcript of muscular dysgenesis (mdg) mice. J Biol Chem 267:25636–25639
Clapham DE (1995) Calcium signaling. Cell 80:259–268
Eu JP, Sun J, Xu L, Stamler JS, Meissner G (2000) The skeletal muscle calcium release channel: coupled O2 sensor and NO signaling functions. Cell 102:499–509
Fairhurst AS (1974) A ryanodine-caffeine-sensitive membrane fraction of skeletal muscle. Am J Physiol 227:1124–1131
Fairhurst AS, Hasselbach W (1970) Calcium efflux from a heavy sarcotubular fraction. Effects of ryanodine, caffeine and magnesium. Eur J Biochem 13:504–509
Fitts RH, Riley DR, Widrick JJ (2001) Functional and structural adaptations of skeletal muscle to microgravity. J Exp Biol 204:3201–3208
Flucher BE, Franzini-Armstrong C (1996) Formation of junctions involved in excitation-contraction coupling in skeletal and cardiac muscle. Proc Natl Acad Sci USA 93:8101–8106
Franzini-Armstrong C, Kenney LJ, Varriano-Marston E (1987) The structure of calsequestrin in triads of vertebrate skeletal muscle: a deep-etch study. J Cell Biol 105:49–56
Franzini-Armstrong C, Protasi F (1997) Ryanodine receptors of striated muscles: a complex channel capable of multiple interactions. Physiol Rev 77:699–729
Furuichi T, Yoshikawa S, Miyawaki A, Wada K, Maeda N, Mikoshiba K (1989) Primary structure and functional expression of the inositol 1,4,5-trisphosphate-binding protein P400. Nature 342:32–38
Kjeldsen K, Richter EA, Galbo H, Lortie G, Clausen T (1986) Training increases the concentration of [3H]ouabain-binding sites in rat skeletal muscle. Biochim Biophys Acta 860:708–712
Kobzik L, Reid MB, Bredt DS, Stamler JS (1994) Nitric oxide in skeletal muscle. Nature 372:546–548
Lee EH, Cherednichenko G, Pessah IN, Allen PD (2006) Functional coupling between TRPC3 and RyR1 regulates the expressions of key triadic proteins. J Biol Chem 281:10042–10048
Maccallini C, Pietrangelo T, Mancinelli R, Amoroso R, Bettoni G, Fulle S (2007) The excitation-contraction coupling on C2C12 skeletal muscle myotubes was modulated by NO-donor ester of gemfibrozil. Nitric Oxide. Dec 15 [Epub ahead of print]
Mackrill JJ, O’Driscoll S, Lai FA, McCarthy TV (2001) Analysis of type 1 ryanodine receptor-12 kDa FK506-binding protein interaction. Biochem Biophys Res Commun 285:52–57
Nakane M, Schmidt HH, Pollock JS, Forstermann U, Murad F (1993) Cloned human brain nitric oxide synthase is highly expressed in skeletal muscle. FEBS Lett 316:175–180
Perkins WJ, Han YS, Sieck GC (1997) Skeletal muscle force and actomyosin ATPase activity reduced by nitric oxide donor. J Appl Physiol 83:1326–1332
Pette D, Staron RS (2001) Transitions of muscle fiber phenotypic profiles. Histochem Cell Biol 115:359–372
Rittweger J, Belavy D, Hunek P, Gast U, Boerst H, Feilcke B, Armbrecht G, Mulder E, Schubert H, Richardson C, de Haan A, Stegeman DF, Schiessl H, Felsenberg D (2006) Highly demanding resistive vibration exercise program is tolerated during 56 days of strict bed-rest. Int J Sports Med 27:553–559
Rudnick J, Puttmann B, Tesch PA, Alkner B, Schoser BG, Salanova M, Kirsch K, Gunga HC, Schiffl G, Luck G, Blottner D (2004) Differential expression of nitric oxide synthases (NOS 1–3) in human skeletal muscle following exercise countermeasure during 12 weeks of bed rest. Faseb J 18:1228–1230
Saborido A, Molano F, Moro G, Megias A (1995) Regulation of dihydropyridine receptor levels in skeletal and cardiac muscle by exercise training. Pflugers Arch 429:364–369
Saito A, Seiler S, Chu A, Fleischer S (1984) Preparation and morphology of sarcoplasmic reticulum terminal cisternae from rabbit skeletal muscle. J Cell Biol 99:875–885
Salanova M, Priori G, Barone V, Intravaia E, Flucher B, Ciruela F, McIlhinney RA, Parys JB, Mikoshiba K, Sorrentino V (2002) Homer proteins and InsP(3) receptors co-localise in the longitudinal sarcoplasmic reticulum of skeletal muscle fibres. Cell Calcium 32:193–200
Soares JM, Duarte JA, Carvalho J, Appell HJ (1993) The possible role of intracellular Ca2+ accumulation for the development of immobilization atrophy. Int J Sports Med 14:437–439
Sorrentino V (1995) The ryanodine receptor family of intracellular calcium release channels. Adv Pharmacol 33:67–90
Sun J, Xin C, Eu JP, Stamler JS, Meissner G (2001a) Cysteine-3635 is responsible for skeletal muscle ryanodine receptor modulation by NO. Proc Natl Acad Sci USA 98:11158–11162
Sun J, Xu L, Eu JP, Stamler JS, Meissner G (2001b) Classes of thiols that influence the activity of the skeletal muscle calcium release channel. J Biol Chem 276:15625–15630
Sun J, Xu L, Eu JP, Stamler JS, Meissner G (2003) Nitric oxide, NOC-12, and S-nitrosoglutathione modulate the skeletal muscle calcium release channel/ryanodine receptor by different mechanisms. An allosteric function for O2 in S-nitrosylation of the channel. J Biol Chem 278:8184–8189
Sutko JL, Airey JA (1996) Ryanodine receptor Ca2+ release channels: does diversity in form equal diversity in function? Physiol Rev 76:1027–1071
Takeshima H, Iino M, Takekura H, Nishi M, Kuno J, Minowa O, Takano H, Noda T (1994) Excitation-contraction uncoupling and muscular degeneration in mice lacking functional skeletal muscle ryanodine-receptor gene. Nature 369:556–559
Xie H, Chen KY, Zhu PH (2004) Effect of Zn2+ ions on ryanodine binding to sarcoplasmic reticulum of striated muscles in the presence of pyrithione. Acta Pharmacol Sin 25:1647–1651
Xu KY, Huso DL, Dawson TM, Bredt DS, Becker LC (1999) Nitric oxide synthase in cardiac sarcoplasmic reticulum. Proc Natl Acad Sci USA 96:657–662
Acknowledgments
We are indebted to Dr. Benedikt G.H. Schoser (LMU Munich, Germany) for providing additional human muscle biopsies and Dr. John Mackrill (UCC Cork, Ireland) for providing the GST-FKBP12 construct. This study was supported by grants from the European Space Agency (ESA), Grant No. 14431/02/NL/SH2), and the German AeroSpace (DLR), Grant No. 50WB0145 (to D.B.). Many thanks to the staff of the Centre for Muscle & Bone Research at CBF, and all participants of the Berlin Bed Rest Study 2003 and 2004. Thanks to Leica Microsystems (http://www.leica-microsystems.com) for technical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salanova, M., Schiffl, G., Rittweger, J. et al. Ryanodine receptor type-1 (RyR1) expression and protein S-nitrosylation pattern in human soleus myofibres following bed rest and exercise countermeasure. Histochem Cell Biol 130, 105–118 (2008). https://doi.org/10.1007/s00418-008-0399-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-008-0399-6