Abstract
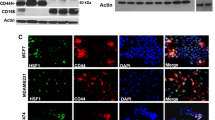
Heat shock proteins (HSPs) play an important role in folding, intracellular localization and degradation of cellular proteins. However, the cellular role of HSP27 is not completely understood. The conflicting results have been reported regarding stress-induced nuclear translocation of HSP27. In this study, human breast cancer cells transiently and stably expressing HSP27–EGFP chimera were utilized to observe the intracellular localization of HSP27. The data show that the transient and stable expression of HSP27–EGFP displayed distinguishingly cellular localization. The nuclear translocalization of HSP27–EGFP was correlated with the presence of G418. Experiments carried out with different human breast cancer cell lines revealed clearly different distribution patterns of endogenous HSP27. The subcellular distribution of endogenous HSP27 appeared diffuse throughout the cytoplasm in MDA435 cells. In MCF-7 and SKBR3 cells, the accumulation of the protein was distinctly seen along the cell membrane and around nucleus. Moreover, the nuclear translocation of endogenous HSP27 was stimulated by G418 only in MDA435 cells, but not in MCF-7 and SKBR3 cells. Overexpression of HSP27 has been associated with resistance to cisplatin and doxorubicin. The correlation of the expression pattern of HSP27 with the drug resistance may need to be investigated. Further studies on the intracellular function of HSP27 may take into account its interaction proteins in the cells. It may provide useful information for the identification of sensitivity of carcinoma cells to the chemotherapeutic drugs and development of more specific agents to circumvent HSP27.






Similar content being viewed by others
References
Billaut-Mulot O, Fernandez-Gomez R, Loyens M, Ouaissi A (1996) Trypanosoma cruzi elongation factor 1-α: nuclear localization in parasites undergoing apoptosis. Gene 174:19–26
Borrelli MJ, Bernock LJ, Landry J, Spitz DR, Weber LA, Hickey E, Freeman ML, Corry PM (2002) Stress protection by a fluorescent Hsp27 chimera that is independent of nuclear translocation or multimeric dissociation. Cell Stress Chaperones 7:281–296
Ciocca DR, Fuqua SA, Lock-Lim S, Toft DO, Welch WJ, McGuire WL (1992) Response of human breast cancer cells to heat shock and chemotherapeutic drugs. Cancer Res 52:3648–3654
Concannon CG, Gorman AM, Samali A (2003) On the role of Hsp27 in regulating apoptosis. Apoptosis 8:61–70
De Jong WW, Caspers GJ, Leunissen JA (1998) Genealogy of the alpha-crystallin small heat-shock protein superfamily. Int J Biol Macromol 22:151–162
Geum D, Son GH, Kim K (2002) Phosphorylation-dependent cellular localization and thermoprotective role of heat shock protein 25 in hippocampal progenitor cells. J Biol Chem 277:19913–19921
Edwards SA, Adamson ED (1987) Isolation of a clone of F9 teratocarcinoma cells “naturally” resistant to G418. J Cell Physiol 133:46–54
Ferrabdi-May E, Cordes V, Biller-Ckovric I, Mirkovic J, Gorlich D, Nicotera P (2001) Caspases mediate nucleoporin cleavage, but not early redistribution of nuclear transport factors and modulation of nuclear permeability in apoptosis. Cell Death Differ 8:495–505
Fuqua SA, Oesterreich S, Hilsenbeck SG, Von Hoff DD, Eckardt J, Osborne CK (1994) Heat shock proteins and drug resistance. Breast Cancer Res Treat 32:67–71
Huot J, Houle F, Spitz R, Landry J (1996) HSP27 phosphorylation-mediated resistance against actin fragmentation and cell death induced by oxidative stress. Cancer Res 56:273–279
Jaattela M (1999) Heat shock proteins as cellular lifeguards. Ann Med 31:261–271
Jin QH, Zhao B, Zhang XJ (2004) Cytochrome c release and endoplasmic reticulum stress are involved in caspase-dependent apoptosis induced by G418. Cell Mol Life Sci 61:1816–1825
Kampinga HH, Brunsting JF, Stege GJ, Landry J, Konings AW, Landry J (1994) Cells overexpressing Hsp27 show accelerated recovery from heat-induced nuclear protein aggregation. Biochem Biophys Res Commun 204:1170–1177
Kato K, Hasegawa K, Goto S, Inaguma Y (1994) Dissociation as a result of phosphorylation of an aggregated form of the small stress protein, hsp27. J Biol Chem 269:11274–11278
Kodiha M, Chu A, Lazrak O, Stochaj U (2005) Stress inhibits nucleocytoplasmic shuttling of heat shock protein hsc70. Am J Physiol Cell Physiol 289:1034–1041
Korber P, Zander T, Herschlag D, Bardwell JC (1999) A new heat shock protein that binds nucleic acids. J Biol Chem 274:249–256
Lavoie JN, Hickey E, Weber LA, Landry J (1993) Modulation of actin microfilament dynamics and fluid phase pinocytosis by phosphorylation of heat shock protein 27. J Biol Chem 268:24210–24214
Lavoie JN, Lambert H, Hickey E, Weber LA, Landry J (1995) Modulation of cellular thermoresistance and actin filament stability accompanies phosphorylation-induced changes in oligomeric structure of heat shock protein 27. Mol Cell Biol 15:505–516
Lindquist S, Craig EA (1988) The heat-shock proteins. Annu Rev Genet 22:631–677
Mearow KM, Dodge ME, Rahimtula M, Yegappan C (2002) Stress-mediated signaling in PC12 cells—the role of the small heat shock protein, Hsp27, and Akt in protecting cells from heat stress and nerve growth factor withdrawal. J Neurochem 83:452–462
Mehlen P, Kretz-Remy C, Briolay J, Fostan P, Mirault ME, Arrigo AP (1995) Intracellular reactive oxygen species as apparent modulators of heat-shock protein 27 (hsp27) structural organization and phosphorylation in basal and tumour necrosis factor alpha-treated T47D human carcinoma cells. Biochem J 312:367–375
Mehlen P, Kretz-Remy C, Preville X, Arrigo AP (1996) Human hsp27, Drosophila hsp27 and human alphaB-crystallin expression-mediated increase in glutathione is essential for the protective activity of these proteins against TNFalpha-induced cell death. EMBO J 15:2695–2706
Miyamoto Y, Saiwaki T, Yamashita J, Yasuda Y, Kotera I, Shibata S, Shigeta M, Hiraoka Y, Haraguchi T, Yoneda Y (2004) Cellular stresses induce the nuclear accumulation of importin α and cause a conventional nuclear import block. J Cell Biol 165:617–623
Muchowski PJ, Bassuk JA, Lubsen NH, Clark JI (1997) Human alphaB-crystallin, small heat shock protein and molecular chaperone. J Biol Chem 272:2578–2582
Oesterreich S, Weng CN, Qiu M, Hilsenbeck SG, Osborne CK, Fuqua SA (1993) The small heat shock protein hsp27 is correlated with growth and drug resistance in human breast cancer cell lines. Cancer Res 53:4443–4448
Parcellier A, Schmitt E, Brunet M, Hammann A, Solary E, Garrido C (2005) Small heat shock proteins HSP27 and alphaB-crystallin: cytoprotective and oncogenic functions. Antioxid Redox Signal 7:404–413
Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llamazares A, Zammanillo D, Hunt T, Nebreda AR (1994) A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell 78:1027–1037
Rust W, Kingsley K, Petnicki T, Padmanabhan S, Carper SW, Plopper GE (1999) Heat shock protein 27 plays two distinct roles in controlling human breast cancer cell migration on laminin-5. Mol Cell Biol Res Commun 1:196–202
Samali A, Orrenius S (1998) Heat shock proteins: regulators of stress response and apoptosis. Cell Stress Chaperones 3:228–236
Shelden EA, Borrelli MJ, Pollock FM, Bonham R (2002) Heat shock protein 27 associates with basolateral cell boundaries in heat-shocked and ATP-depleted epithelial cells. J Am Soc Nephrol 13: 332–341
Stahl J, Bielka H (1983) Present conceptions on the peptidyltransferase centre of animal ribosomes. Biomed Biochim Acta 41:47–55
Takayama S, Reed JC, Homma S (2003) Heat-shock proteins as regulators of apoptosis. Oncogene 22:9041–9047
Thanner F, Sutterlin MW, Kapp M, Rieger L, Morr AK, Kristen P, Dietl J, Gassel AM, Muller T (2005) Heat shock protein 27 is associated with decreased survival in node-negative breast cancer patients. Anticancer Res 25:1649–1653
Tsuchiya A, Tashiro E, Yoshida M, Imoto M (2005) Involvement of nuclear accumulation of heat shock protein 27 in leptomycin B-induced apoptosis in Hela cells. J Antibiot (Tokyo) 58:810–816
Vargas-Roig LM, Fanelli MA, Lopez LA, Gago FE, Tello O, Aznar JC, Ciocca DR (1997) Heat shock proteins and cell proliferation in human breast cancer biopsy samples. Cancer Detect Prev 21:441–451
Vargas-Roig LM, Gago FE, Tello O, Aznar JC, Ciocca DR (1998) Heat shock protein expression and drug resistance in breast cancer patients treated with induction chemotherapy. Int J Cancer 79:468–475
Vayssier M, Polla BS (1998) Heat shock proteins chaperoning life and death. Cell Stress Chaperones 3:221–227
Wang HP, Hanlon JG, Rainbow AJ, Espiritu M, Singh G (2002) Up-regulation of Hsp27 plays a role in the resistance of human colon carcinoma HT29 cells to photooxidative stress. Photochem Photobiol 76:98–104
Xanthoudakis S, Nicholson DW (2000) Heat-shock proteins as death determinants. Nat Cell Biol 2:E163–E165
Yamamoto K, Okamoto A, Isonishi S, Ochiai K, Ohtake Y (2001) Heat shock protein 27 was up-regulated in cisplatin resistant human ovarian tumor cell line and associated with the cisplatin resistance. Cancer Lett 168:173–181
Acknowledgment
This work was supported by Beijing Natural Science Foundation (No. 7051006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qian, L., Zhang, Z., Shi, M. et al. Expression and distribution of HSP27 in response to G418 in different human breast cancer cell lines. Histochem Cell Biol 126, 593–601 (2006). https://doi.org/10.1007/s00418-006-0195-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-006-0195-0