Abstract
Purpose
This study was aimed at exploring the function of Exosomes isolated from bone marrow-derived mesenchymal stem cells (BMSC-Exos) in corneal wound healing and at revealing the underlying mechanisms involving the p44/42 mitogen-activated protein kinase (MAPK) pathway.
Methods
The isolated BMSC-Exos were identified by transmission electron microscopy, Western blot, and nanoparticle tracking analysis. After coculture with BMSC-Exos, the proliferation and migration of human corneal epithelial cells (HCEs) were evaluated. The protein expression of p-MEK/MEK and p44/42 MAPK was detected by Western blot. A mouse model of alkali-burned cornea was established via NaOH exposure. After injection with BMSC-Exos, the pathological changes and expression of α-SMA (a fibrosis marker) and CD31 (a vascularization marker) in corneal tissues were detected.
Results
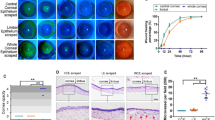
BMSC-Exos enhanced the proliferation and migration of HCEs in a dose-dependent manner. The p44/42 MAPK pathway was activated by the treatment of BMSC-Exos, and its blocking using U0126 partially abrogated the effects of BMSC-Exos on promoting the proliferation and migration of HCEs. In vivo, the injection of BMSC-Exos facilitated the remission of the pathological changes (inflammation) and weakened the upregulation of α-SMA (fibrosis) and CD31 (vascularization) in corneal tissues of mice with alkali-burn injury.
Conclusion
BMSC-Exos promoted the proliferation and migration of HCEs via activating the p44/42 MAPK pathway in vitro and also inhibited alkali burn-induced inflammation, fibrosis, and vascularization in corneal tissues in vivo. BMSC-Exos may be promising resources for promoting corneal wound healing.






Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Notara M, Alatza A, Gilfillan J, Harris AR, Levis HJ, Schrader S, Vernon A, Daniels JT (2010) In sickness and in health: corneal epithelial stem cell biology, pathology and therapy. Exp Eye Res 90(2):188–195. https://doi.org/10.1016/j.exer.2009.09.023
Bremond-Gignac D, Copin H, Benkhalifa M (2018) Corneal epithelial stem cells for corneal injury. Expert Opin Biol Ther 18(9):997–1003. https://doi.org/10.1080/14712598.2018.1508443
Ljubimov AV, Saghizadeh M (2015) Progress in corneal wound healing. Prog Retin Eye Res 49:17–45. https://doi.org/10.1016/j.preteyeres.2015.07.002
Barrientez B, Nicholas SE, Whelchel A, Sharif R, Hjortdal J, Karamichos D (2019) Corneal injury: clinical and molecular aspects. Exp Eye Res 186:107709. https://doi.org/10.1016/j.exer.2019.107709
Kalluri R (2016) The biology and function of exosomes in cancer. J Clin Invest 126(4):1208–1215. https://doi.org/10.1172/jci81135
Braicu C, Tomuleasa C, Monroig P, Cucuianu A, Berindan-Neagoe I, Calin GA (2015) Exosomes as divine messengers: are they the Hermes of modern molecular oncology? Cell Death Differ 22(1):34–45. https://doi.org/10.1038/cdd.2014.130
Lee BR, Kim JH, Choi ES, Cho JH, Kim E (2018) Effect of young exosomes injected in aged mice. Int J Nanomedicine 13:5335–5345. https://doi.org/10.2147/ijn.S170680
Phinney DG, Pittenger MF (2017) Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells 35(4):851–858. https://doi.org/10.1002/stem.2575
Furlani D, Ugurlucan M, Ong L, Bieback K, Pittermann E, Westien I, Wang W, Yerebakan C, Li W, Gaebel R, Li RK, Vollmar B, Steinhoff G, Ma N (2009) Is the intravascular administration of mesenchymal stem cells safe? Mesenchymal stem cells and intravital microscopy. Microvasc Res 77(3):370–376. https://doi.org/10.1016/j.mvr.2009.02.001
Villarroya-Beltri C, Baixauli F, Gutiérrez-Vázquez C, Sánchez-Madrid F, Mittelbrunn M (2014) Sorting it out: regulation of exosome loading. Semin Cancer Biol 28:3–13. https://doi.org/10.1016/j.semcancer.2014.04.009
Shen Z, Huang W, Liu J, Tian J, Wang S, Rui K (2021) Effects of mesenchymal stem cell-derived exosomes on autoimmune diseases. Front Immunol 12:749192. https://doi.org/10.3389/fimmu.2021.749192
Zhu W, Huang L, Li Y, Zhang X, Gu J, Yan Y, Xu X, Wang M, Qian H, Xu W (2012) Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo. Cancer Lett 315(1):28–37. https://doi.org/10.1016/j.canlet.2011.10.002
Vallabhaneni KC, Penfornis P, Xing F, Hassler Y, Adams KV, Mo YY, Watabe K, Pochampally R (2017) Stromal cell extracellular vesicular cargo mediated regulation of breast cancer cell metastasis via ubiquitin conjugating enzyme E2 N pathway. Oncotarget 8(66):109861–109876. https://doi.org/10.18632/oncotarget.22371
Favaro E, Carpanetto A, Caorsi C, Giovarelli M, Angelini C, Cavallo-Perin P, Tetta C, Camussi G, Zanone MM (2016) Human mesenchymal stem cells and derived extracellular vesicles induce regulatory dendritic cells in type 1 diabetic patients. Diabetologia 59(2):325–333. https://doi.org/10.1007/s00125-015-3808-0
Shen T, Zheng QQ, Shen J, Li QS, Song XH, Luo HB, Hong CY, Yao K (2018) Effects of adipose-derived mesenchymal stem cell exosomes on corneal stromal fibroblast viability and extracellular matrix synthesis. Chin Med J (Engl) 131(6):704–712. https://doi.org/10.4103/0366-6999.226889
Wang S, Hou Y, Li X, Song Z, Sun B, Li X, Zhang H (2020) Comparison of exosomes derived from induced pluripotent stem cells and mesenchymal stem cells as therapeutic nanoparticles for treatment of corneal epithelial defects. Aging (Albany NY) 12(19):19546–19562. https://doi.org/10.18632/aging.103904
Samaeekia R, Rabiee B, Putra I, Shen X, Park YJ, Hematti P, Eslani M, Djalilian AR (2018) Effect of human corneal mesenchymal stromal cell-derived exosomes on corneal epithelial wound healing. Invest Ophthalmol Vis Sci 59(12):5194–5200. https://doi.org/10.1167/iovs.18-24803
Garbison KE, Heinz BA, Lajiness ME (2004) Phospho-ERK Assays. In: Markossian S, Grossman A, Brimacombe K et al (eds) Assay Guidance Manual. Eli Lilly & Company and the National Center for Advancing Translational Sciences, Bethesda (MD)
Roy Chowdhury U, Bahler CK, Holman BH, Fautsch MP (2017) ATP-sensitive potassium (KATP) channel openers diazoxide and nicorandil lower intraocular pressure by activating the Erk1/2 signaling pathway. PLoS One 12(6):e0179345. https://doi.org/10.1371/journal.pone.0179345
Jiang D, Liu X, Hu J (2017) Topical administration of Esculetin as a potential therapy for experimental dry eye syndrome. Eye (Lond) 31(12):1724–1732. https://doi.org/10.1038/eye.2017.117
Park JH, Moon SH, Kang DH, Um HJ, Kang SS, Kim JY, Tchah H (2018) Diquafosol sodium inhibits apoptosis and inflammation of corneal epithelial cells via activation of Erk1/2 and RSK: in vitro and in vivo dry eye model. Invest Ophthalmol Vis Sci 59(12):5108–5115. https://doi.org/10.1167/iovs.17-22925
Nagai N, Fukuoka Y, Ishii M, Otake H, Yamamoto T, Taga A, Okamoto N, Shimomura Y (2018) Instillation of sericin enhances corneal wound healing through the ERK pathway in rat debrided corneal epithelium. Int J Mol Sci 19(4). https://doi.org/10.3390/ijms19041123
Jeong WY, Yoo HY, Kim CW (2017) Neuregulin-1 accelerates corneal epithelial wound healing. Growth Factors 35(6):225–233. https://doi.org/10.1080/08977194.2018.1436055
Lan Y, Kodati S, Lee HS, Omoto M, Jin Y, Chauhan SK (2012) Kinetics and function of mesenchymal stem cells in corneal injury. Invest Ophthalmol Vis Sci 53(7):3638–3644. https://doi.org/10.1167/iovs.11-9311
Lin KJ, Loi MX, Lien GS, Cheng CF, Pao HY, Chang YC, Ji AT, Ho JH (2013) Topical administration of orbital fat-derived stem cells promotes corneal tissue regeneration. Stem Cell Res Ther 4(3):72. https://doi.org/10.1186/scrt223
Tao H, Chen X, Cao H, Zheng L, Li Q, Zhang K, Han Z, Han ZC, Guo Z, Li Z, Wang L (2019) Mesenchymal stem cell-derived extracellular vesicles for corneal wound repair. Stem Cells Int 2019:5738510. https://doi.org/10.1155/2019/5738510
Yeung V, Zhang TC, Yuan L, Parekh M, Cortinas JA, Delavogia E, Hutcheon AEK, Guo X, Ciolino JB (2022) Extracellular vesicles secreted by corneal myofibroblasts promote corneal epithelial cell migration. Int J Mol Sci 23(6). https://doi.org/10.3390/ijms23063136
Yeung V, Boychev N, Farhat W, Ntentakis DP, Hutcheon AEK, Ross AE, Ciolino JB (2022) Extracellular Vesicles in Corneal Fibrosis/Scarring. Int J Mol Sci 23(11). https://doi.org/10.3390/ijms23115921
Teng X, Chen L, Chen W, Yang J, Yang Z, Shen Z (2015) Mesenchymal stem cell-derived exosomes improve the microenvironment of infarcted myocardium contributing to angiogenesis and anti-inflammation. Cell Physiol Biochem 37(6):2415–2424. https://doi.org/10.1159/000438594
de Oliveira RC, Wilson SE (2020) Fibrocytes, wound healing, and corneal fibrosis. Invest Ophthalmol Vis Sci 61(2):28. https://doi.org/10.1167/iovs.61.2.28
Torricelli AA, Santhanam A, Wu J, Singh V, Wilson SE (2016) The corneal fibrosis response to epithelial-stromal injury. Exp Eye Res 142:110–118. https://doi.org/10.1016/j.exer.2014.09.012
Biber JM, Holland EJ, Neff KD (2010) Management of ocular stem cell disease. Int Ophthalmol Clin 50(3):25–34. https://doi.org/10.1097/IIO.0b013e3181e20d64
Shu DY, Lovicu FJ (2017) Myofibroblast transdifferentiation: The dark force in ocular wound healing and fibrosis. Prog Retin Eye Res 60:44–65. https://doi.org/10.1016/j.preteyeres.2017.08.001
Ju B, Guo O, Benissan-Messan DZ, Shawver MH, Chen P, Geng B, Wei S, Yaron JR, Lucas AR, Zhu H (2021) Serp-1 promotes corneal wound healing by facilitating re-epithelialization and inhibiting fibrosis and angiogenesis. Front Cardiovasc Med 8:649124. https://doi.org/10.3389/fcvm.2021.649124
Roskoski R Jr (2012) ERK1/2 MAP kinases: structure, function, and regulation. Pharmacol Res 66(2):105–143. https://doi.org/10.1016/j.phrs.2012.04.005
Yang HM, Kang SW, Sung J, Kim K, Kleinman H (2020) Purinergic signaling involvement in thymosin β4-mediated corneal epithelial cell migration. Curr Eye Res 45(11):1352–1358. https://doi.org/10.1080/02713683.2020.1748891
Mediero A, Guzmán-Aranguez A, Crooke A, Peral A, Pintor J (2008) Corneal re-epithelialization stimulated by diadenosine polyphosphates recruits RhoA/ROCK and ERK1/2 pathways. Invest Ophthalmol Vis Sci 49(11):4982–4992. https://doi.org/10.1167/iovs.07-1583
Giurdanella G, Longo A, Salerno L, Romeo G, Intagliata S, Lupo G, Distefano A, Platania CBM, Bucolo C, Li Volti G, Anfuso CD, Pittalà V (2021) Glucose-impaired corneal re-epithelialization is promoted by a novel derivate of dimethyl fumarate. Antioxidants (Basel) 10(6). https://doi.org/10.3390/antiox10060831
Zhu G, Pei L, Lin F, Yin H, Li X, He W, Liu N, Gou X (2019) Exosomes from human-bone-marrow-derived mesenchymal stem cells protect against renal ischemia/reperfusion injury via transferring miR-199a-3p. J Cell Physiol 234(12):23736–23749. https://doi.org/10.1002/jcp.28941
Funding
This work was supported by the Natural Science Foundation of China (No. 81200666).
Author information
Authors and Affiliations
Contributions
Jin Zhou: conceptualization; Yongqiang Zhang: methodology; Dehui Zheng and Lifeng Yan: formal analysis and investigation; Jin Zhou and Yuanyuan Ding: writing—original draft preparation; Mengxiang Guo and Yongqiang Zhang: writing—review and editing; Jin Zhou: funding acquisition; Yani Mao and Lihong Yang: resources; Yuanyuan Ding: supervision.
Corresponding author
Ethics declarations
Ethics approval
This study complied with the National Institutes of Health's guidelines for the care and use of laboratory animals. This study was approved by the Animal Ethics Committee of Guangzhou Women and Children’s Medical Center.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, J., Ding, Y., Zhang, Y. et al. Exosomes from bone marrow-derived mesenchymal stem cells facilitate corneal wound healing via regulating the p44/42 MAPK pathway. Graefes Arch Clin Exp Ophthalmol 261, 723–734 (2023). https://doi.org/10.1007/s00417-022-05956-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-022-05956-4