Abstract
Purpose
Retinal alterations in inherited metabolic diseases associated with neurodegeneration are poorly studied. The objective was to study retinal thickness, specifically the components of the ganglion cell complex (GCC)—nerve fiber layer (NFL), ganglion cell layer (GCL), and inner plexiform layer (IPL)—using spectral-domain optical coherence tomography (SD-OCT) in two different diseases with potential dopaminergic depletion, phenylketonuria (PKU) and Gaucher disease type 3 (GD3).
Methods
Retinal layers in 19 patients with PKU, 15 patients with GD3, and 93 healthy individuals were measured using peripapillary ring scan and macular SD-OCT. Linear mixed models were computed including an adjustment for age, sex, and spherical equivalent. We calculated Spearman’s rank correlations between retinal layer measurements and clinical and/or laboratory parameters.
Results
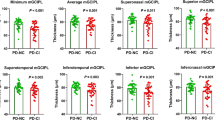
Thinning of total retinal thickness was found in the macular inner ring (p = 0.002), and outer ring (p = 0.012), sparing the fovea (p = 0.12) in PKU, while in GD3, all subfields were thinned (fovea p < 0.001, inner ring p = 0.047, outer ring 0.07). In both conditions, thinning was most evident in the NFL, GCL, and IPL, while OPL (outer plexiform layer) was thickened. Peripapillary retinal nerve fiber layer measurements remained normal. GCL and IPL in PKU correlated with tyrosine serum concentration.
Conclusion
Thinning of the NFL, GCL, and IPL, with thickened OPL, are both found in PKU and in GD3. Low dopamine concentrations in the retina might promote these effects. However, these data do not give evidence that retinal measurements can be used as a biomarker for disease severity in patients with GD3.

Similar content being viewed by others
Introduction
Optical coherence tomography (OCT), a non-invasive technique with a high resolution, enables to describe retinal structures almost at a cellular level in vivo. OCT layers correlate well with histological findings of the retina [1, 2]. The use of this imaging technique to demonstrate neurodegeneration of the retina or the optic disc in neurological and neuro-ophthalmological conditions is growing [3]. Retinal correlates have been proved primarily in Parkinson’s disease, Alzheimer’s disease, and multiple sclerosis, but also in rare conditions such as amyotrophic lateral sclerosis, and Huntington’s disease [3,4,5]. They resemble changes found in glaucoma by thinning of the macular ganglion cell complex [6], defined by the three innermost retinal layers (nerve fiber layer, NFL; ganglion cell layer, GCL; and inner plexiform layer, IPL) [7]. On the one hand, thinning of the combined ganglion cell-inner plexiform layer (GCIPL) and thinning of the peripapillary retinal nerve fiber layer (pRNFL) correlate with neurodegenerative processes of the entire CNS, such as in multiple sclerosis [3, 4], or with nigrostriatal dopaminergic degeneration related to Parkinson’s disease [8]. On the other hand, retinal layer measurements may provide structural evidence for dysfunction in the fovea and parafovea and retinal dopamine loss, as is assumed in Parkinson’s disease [8,9,10]. The retina is one of the tissues in the body with highest dopamine concentration [11]. Dopamine receptors are expressed in retinal cells throughout the retina with varying functions depending on the receptor subtype and the cell type [12]. The retinal dopaminergic system is involved in eye growth, light adaptation, circadian rhythmicity, and cell survival [13]. Dopamine has an important role in uncoupling horizontal and amacrine cell junctions [12]. Dopaminergic amacrine cells are located in the inner nuclear layer (INL) [14, 15].
Ganglion cell complex (GCC) thinning has recently been reported for the first time in the inherited metabolic diseases phenylketonuria[16] and Gaucher disease type 3 [17]. In both conditions, a dopaminergic depletion can be speculated. In PKU, dopamine depletion has been related to reduced tyrosine uptake into the brain and reduced tyrosine-hydroxylase activity [18]. In Gaucher type 1, GCC thinning was demonstrated primarily in the presence of parkinsonian features or other clinical markers of early neurodegeneration (hyposmia, cognitive impairment, parkinsonian motor signs) [17, 19, 20]. Thus, in both diseases, changes in neurotransmission may be one mechanism leading to functional and morphological retina alteration.
Our aim was to study segmental retinal layers, specifically the components of the ganglion cell complex (GCC)—nerve fiber layer (NFL), ganglion cell layer (GCL), and inner plexiform layer (IPL)—by means of spectral-domain (SD) optical coherence tomography (OCT) in two different diseases with potential dopaminergic depletion, phenylketonuria (PKU) and Gaucher disease type 3 (GD3). The second aim was to identify whether retinal layer measurements correlate with established disease features and if they may be used as a biomarker for disease severity. This should be of special interest for patients with GD3.
Methods
Study population
This study included 19 patients aged 6 to 46 years with mild, or classical, phenylketonuria or tetrahydrobiopterin-deficient hyperphenylalaninemia, 15 patients aged 6 to 44 years with Gaucher disease type 3, and 93 controls aged 6 to 75 years. The patients’ inclusion criteria were genetical and/or biochemical prove of their disease. Participants under 6 years of age were excluded. Controls were included if they had no ocular disease and age-appropriate visual function, as well as no relevant systemic disease (e.g., neurodegeneration). Controls were recruited stratifying for age (seven subgroups with at least 10 participants were built for an even age distribution).
The study was approved by the Medical Ethical Committee of the State Chamber of Medicine of Rhineland Palatinate in Mainz, Germany (reference number 837.373.14). All persons or their parents/guardians gave their written informed consent prior to inclusion in the study. The research adhered to the tenets of the Declaration of Helsinki.
Ophthalmic examination procedure
The examination included non-cycloplegic auto-refraction measurements (NIDEK AR-360A, Nidek Co., Japan), best-corrected visual acuity testing, slit lamp biomicroscopy, and fundus examination, as well as orthoptic examination, which were published elsewhere [21,22,23]. Spherical equivalents defined as the sum of the spherical power and half of the cylindric power were used in the statistic models.
Imaging of the optic nerve head and the macula was carried out using spectral-domain (SD) optical coherence tomography (OCT) (Spectralis, Heidelberg Engineering GmbH, Heidelberg, Germany) with automatic real-time function for image averaging. We acquired a peripapillary OCT and a macular OCT. The peripapillary retinal nerve fiber layer (pRNFL) was imaged with a diameter of 12° (corresponding to 3.47 mm in the standard eye), and a standard corneal curvature of 7.7 mm. For the macular OCT, 49 horizontal single scans were acquired. After semi-automated segmentation of the retinal layers as provided by the OCT software (Heidelberg Eye Explorer version 1.10.2.0, viewing module 6.9.5.0; HEYEX, Heidelberg, Germany), all scans were assessed regarding their quality by a board-certified ophthalmologist (SH). Those with segmentation errors were corrected, or excluded in cases of poor image quality. In cases of poor data in only one sector, this sector was excluded prior to analysis; if more sectors were affected, then the complete OCT dataset of this eye was excluded.
Mean retinal thickness of 9 macular layers was used for the analysis: total retinal layer thickness of the macula, nerve fiber layer (NFL), ganglion cell layer (GCL), inner plexiform layer (IPL), inner nuclear layer (INL), outer plexiform layer (OPL), outer nuclear layer (ONL), outer retinal layer (ORL) (being limited by the external limiting membrane and Bruch’s membrane, this layer corresponds to the photoreceptors), and retinal pigment epithelium (RPE).
The 6-mm macular scan measurements were classified according to the ETDRS segments (“Early Treatment Diabetic Retinopathy Study” subfields). Central zone, inner ring, and outer ring with diameters of 1, 3, and 6 mm, respectively, were included in the analysis. The average of all points within the central zone (1 mm diameter) was defined as foveal thickness, the inner ring (1 to 3 mm) as parafoveal thickness, and the outer ring (3 to 6 mm) as perifoveal thickness.
Clinical data
Typical variables described for disease stage in PKU and GD3 were determined and obtained from the patient’s records. For PKU, we used first, current phenylalanine serum concentrations of each individual (mean 693 µmol/l ± 384 µmol/l); second, current tyrosine serum concentrations of each individual (mean 105 µmol/l ± 60 µmol/l); third, disease treatment (16 early-treated vs. three late-treated individuals with PKU); and fourth, disease type (mild phenylketonuria with untreated blood phenylalanine concentrations of less than 1000–1200 µmol/l) [24], classical phenylketonuria, and tetrahydrobiopterin-deficient hyperphenylalaninemia). For GD3, we used first, the modified severity scoring tool (mSST), which is based on twelve domains including horizontal gaze palsy, cranial nerve palsy, seizures and age at first seizures, cognitive ability, ataxia, tremor, spasticity, rigidity, dysphagia, dysarthria, and spinal alignment [25]. Second, we considered the phenotype severity (mild, intermediate, severe), of which intermediate phenotype was associated with homozygous L444P mutation [22]. Third/fourth, we included horizontal/vertical peak velocity of reflexive saccades in GD3 (69°/s ± 58°/s and 192°/s ± 92°/s, respectively).
Statistical analysis
Medians, interquartile ranges, minimums, and maximums were calculated for all continuous variables. For variables distributed normally, means and standard deviations were computed. For dichotomous variables, absolute and relative frequencies were computed.
To analyze the differences of retinal thickness with respect to PKU and GD3, we used linear mixed models to control for the inclusion of one and two eyes of a study participant (as random effect). A further adjustment for age, sex, and spherical equivalent was included in the statistical analysis. Full thickness measurements are more susceptible to these parameters, than single layer measurements, which is why we focused on single layer correlation analysis as follows. Spearman’s rank correlation was conducted to correlate thinned or thickened layers (e.g., GCL or OPL, respectively) with disease-specific variables of PKU (current phenylalanine and tyrosine serum concentration, early- vs. late-treated PKU, and disease type) and GD3 (mSST, phenotype severity, horizontal and vertical eye movements). The other retinal layers, which were not different to controls, or which were underrepresented in the specific subfield (fovea), were not further analyzed. Correlation coefficient rho of ≥ 0.5 was regarded a moderate correlation, correlation coefficient of ≥ 0.3 was considered a weak correlation, and < 0.3 was considered a no correlation. Statistical analysis was performed using R version 4.0.4. All p-values should be regarded as continuous parameters that reflect the level of evidence from our explorative analysis and are therefore reported exactly.
Results
All patients examined were included in the study. From the 19 PKU patients (mean age 20 ± 12 years), 15 GD3 patients (mean age 20 ± 10 years), and 93 controls (mean age 32 ± 17 years), we excluded 6, 8, and 8 eyes respectively for the pRNFL analysis due to poor image quality, and 0, 2, and 6 eyes respectively for the macular OCT analysis.
Macular full thickness and macular layers
Phenylketonuria
The OCT measurements indicate a significant thinner total retinal thickness in the inner and outer ring, but not in the fovea. This pattern affected the NFL, GCL, IPL, and ONL, while OPL was thickened. The differences were most evident in the NFL, GCL, and IPL (see Table 1) and more evident than in GD3. The remaining layers (INL, and ORL) did not differ from controls. RPE was thinned in the inner ring segment, but the difference was not significant after adjustment for sex, age, and spherical equivalent.
GCL correlated with current tyrosine serum concentration (outer ring: rho = 0.70, p = 0.0008 (< 0.001); inner ring: rho = 0.51, p = 0.025), as well as IPL did (inner ring: rho = 0.61, p = 0.006). ONL correlated inversely with current phenylalanine serum concentration (outer ring: rho = − 0.59, p = 0.01; inner ring: − 0.63, p = 0.004). Early-treated PKU patients had rather thick GCL (inner ring: rho = 0.55, p = 0.014) and thick IPL (inner ring: rho = 0.54, p = 0.02) compared to late-treated patients.
Gaucher disease type 3
Thinning of total retinal thickness was found in all subfields (fovea, inner ring, and outer ring) compared to the controls, even after adjusting for sex, age, and spherical equivalent (Table 1). The retinal layers affected by thinning were NFL (outer ring significantly), GCL (inner ring significantly), and IPL (slightly), while OPL (outer ring) was thickened, and the remaining layers (INL, ONL, ORL, and RPE) showed no difference in thickness to the controls. Individual foveal layers were normal (when only considering the layers ONL outwards, because the layers NFL to OPL are very small and do not yield reliable OCT data in general). However, total retinal thinning was most evident in the fovea.
NFL outer ring correlated inversely with mSST (outer ring: rho = − 0.49; p = 0.046; inner ring: rho = − 0.39, p = 0.11), and OPL correlated inversely with horizontal peak velocity (OPL outer ring: rho = − 0.57, p = 0.020; OPL inner ring: rho = − 0.51, p = 0.040). In GD3, no correlations were found for foveal total retinal thickness, and GCL, and none with vertical peak velocity or phenotype severity (mild/intermediate/severe).
Peripapillary RNFL
The global peripapillary RNFL did not differ significantly between the groups with 96 µm in PKU and GD3 and 96.7 µm in control eyes. Regarding the distinct peripapillary RNFL sectors, only the temporal-inferior sector was significantly thicker in PKU eyes compared to controls (p = 0.029), but the difference did not remain significant after adjusting for sex, age, and spherical equivalent (Table 2). The other sectors were normal in both diseases.
Discussion
This study is one of the first, reporting OCT measurements in phenylketonuria and Gaucher disease type 3, besides the publications from Serfozo et al. [16, 26] and Tantawy et al. [17]. Our main finding, GCC reduction, is in line with these two studies [16, 17, 26]. We additionally demonstrate that each of the individual components (NFL, GCL, and IPL) is reduced in thickness in phenylketonuria. A similar pattern of retinal thinning was evident in GD3. The retinal measurements found in both conditions resemble changes seen during aging: GCL and IPL both thin out, while OPL thickens with age [27].
Macular thickness
Alterations of the ganglion cell complex in various neurodegenerative diseases overlap and tend to show similarities. We found GCC reduction both in PKU and in GD3.
Phenylketonuria
Our data support the findings of Serfozo et al., who reported total retinal thinning in the parafoveal and perifoveal region, sparing the fovea [26], in early-treated phenylketonuria, and reduced GCC thickness (average, superior, and inferior quadrants) [16]. We further demonstrated OPL thickening in the inner and outer ring. Inverse correlation of phenylalanine serum concentration with retinal measurements was, at most, inconsistently found [26]. We found tyrosine serum concentrations correlating with GCL and IPL, which might indirectly indicate low cerebral tyrosine and dopamine concentrations.
Gaucher disease
In line with previous investigations, we confirm significant retinal thinning in the NFL (outer ring), GCL (outer and inner ring), and IPL (inner ring) after adjusting for sex, age, and refraction, and thickening of the OPL in GD3. Our data thus attest reduced GCC as reported by Tantawy et al., who divided a cohort of GD patients aged 11 to 29 years into a group with parkinsonian features (n = 11) and a group without (n = 37), independently of the type of GD. Their results were that GCC thickness differed between young patients with parkinsonian features and those without parkinsonian features. However, between GD1 (n = 14) and GD3 (n = 34), GCC thickness did not differ significantly, although all GD patients together (93.1 (± 7.0) µm) differed from the controls (98.7 (± 9.6) µm) [17]. Similarly, thinning of the retinal GCC was associated with potential clinical markers of early neurodegeneration in GD1 (n = 11) or GBA mutation carriers (intermediate level of glucocerebrosidase activity) in a study conducted by McNeill et al. [20]. GD1 patients without neurodegenerative symptoms do not show retinal GCC thinning [19, 20], although subsections of the GCC thickness (e.g., of the outer macular GCC nasally and inferiorly) revealed significant thinning [19]. In GD1, no correlation was found between retinal measurements and disease severity [19]. In GD3, we detected a weak correlation between modified severity scoring tool, and retinal NFL, and no correlation between saccadic measurements and retinal layer measurements.
The pathways of retinal damage including that of retinal ganglion cells are not fully understood in both diseases. Changes in neurotransmitter metabolism are discussed to play a role. Retinal dopamine deficiency is discussed to play a role in primary retina degeneration and secondary loss of dopamine-regulated neurons [26]. Besides disturbances in neurotransmitter metabolism, other changes may lead to morphological changes of the retina in both disorders. In PKU, this might be a direct neurotoxic effect of phenylalanine [28]. In the lysosomal storage disorder GD, the reduced activity of β-glucocerebrosidase is associated with accumulation of α-synuclein, inhibition of apoptosis, and reduced mitochondrial function with associated oxidative stress [29, 30]. Clinically, vitreous fluid of GD3 patients may contain visible opacities as well as Gaucher cells, and high concentration of glucosylceramide [31, 32]. In addition, vascular abnormalities with tortuosity, and occlusion were discussed to induce retinal damage [19]. In the present cohort, only three patients presented increased tortuosity of retinal vessels bilaterally, one patient presented caliper changes of retinal vessels, and two patients showed peripapillary atrophy of the outer retina [22].
As follow-up data are missing, we cannot exclude that in GD3 metabolic imbalances during embryonic development already influence a normal retina development.
Peripapillary RNFL
Phenylketonuria
While Serfozo et al. found that average pRNFL was reduced—without prove of reduction in the quadrants—in early-treated phenylketonuria [16], we did not detect reduction in pRNFL thickness. The values of our pRNFL data, however, are in line with those measured by Serfozo et al., namely 96.3 µm (± 9.9 µm) in early-treated phenylketonuria. The different outcome might be attributed to thicker pRNFL in their controls (101.9 ± 7.2 µm) compared to ours [16].
Gaucher disease
Peripapillary RNFL reduction as detected by Matos et al. in a GD3 patient is not confirmed by our data. In this case, the authors attributed their findings to either glaucoma of normal pressure or a nervous degeneration [33]. While no other studies have investigated pRNFL in GD3, Weill et. al reported abnormal pRNFL scans in one-third of GD1 patients with significant thinning of the average, superior, and inferior pRNFL [19]. This pattern of damage was supposed to match a magnocellular type, which is also characteristic in Alzheimer’s disease [34]. From our data, we cannot conclude a damage or a pattern of damage at the level of the optic disc primarily due to GD3.
Limitations and perspectives
This study has several limitations. First, we did not have complete long-term blood biomarker data of the PKU group (mean phenylalanine serum concentrations over the past 5 or 10 years) for the analysis. We also could not provide data of parkinsonian motor signs (bradykinesia, rigidity, rest tremor) [17], or prodromal symptoms (hyposmia, cognitive impairment, hallucinations, depression, sleep disorders, and autonomic dysfunction) [20] in the GD3 group. Unfortunately, these data were not available in our cohort. Longitudinal data is still missing, which could elucidate whether progression occurs over time. This is even more important, as progression in neuro-ophthalmologic diseases is subject to high interindividual variation.
Conclusion
This OCT study with PKU and GD3 patients confirmed that retinal thickness is reduced, to elucidate, at the level of the NFL, GCL, IPL, and ONL, while OPL is thickened in PKU patients. The same is held true for GD3 although with fewer significance. Individual follow-up examinations are required for evaluation and detection of a progression of retinal neurodegeneration. This is important, because current therapies for these conditions might interfere with progressive morphological changes of the retina.
Data availability
The data are included in the manuscript. Participants of the study did not agree for their individual raw dataset to be shared publicly, so supporting data is not available.
Change history
28 February 2022
The original version of this paper was updated to add the missing compact agreement Open Access funding note.
References
Moreira-Neto CA, Bergeron S, Coblentz J et al (2019) Optimizing optical coherence tomography and histopathology correlation in retinal imaging. Can J Ophthalmol 54:280–287. https://doi.org/10.1016/j.jcjo.2018.06.024
Xie W, Zhao M, Tsai S-H et al (2018) Correlation of spectral domain optical coherence tomography with histology and electron microscopy in the porcine retina. Exp Eye Res 177:181–190. https://doi.org/10.1016/j.exer.2018.08.003
Papadopoulou A, Oertel FC, Zimmermann H et al (2018) Optical coherence tomography in disorders of the central nervous system. Klin Monbl Augenheilkd 235:1242–1258. https://doi.org/10.1055/a-0715-7961
Alonso R, Gonzalez-Moron D, Garcea O (2018) Optical coherence tomography as a biomarker of neurodegeneration in multiple sclerosis: a review. Mult Scler Relat Disord 22:77–82. https://doi.org/10.1016/j.msard.2018.03.007
Yap TE, Balendra SI, Almonte MT, Cordeiro MF (2019) Retinal correlates of neurological disorders. Ther Adv Chronic Dis 10:2040622319882205. https://doi.org/10.1177/2040622319882205
Chrysou A, Jansonius NM, van Laar T (2019) Retinal layers in Parkinson’s disease: a meta-analysis of spectral-domain optical coherence tomography studies. Parkinsonism Relat Disord 64:40–49. https://doi.org/10.1016/j.parkreldis.2019.04.023
Tan O, Chopra V, Lu AT-H et al (2009) Detection of macular ganglion cell loss in glaucoma by Fourier-domain optical coherence tomography. Ophthalmology 116:2305-2314.e2. https://doi.org/10.1016/j.ophtha.2009.05.025
Lee J-Y, Ahn J, Shin JY, Jeon B (2021) Parafoveal change and dopamine loss in the retina with Parkinson’s disease. Ann Neurol 89:421–422. https://doi.org/10.1002/ana.25972
Lee J-Y, Ahn J, Kim TW, Jeon BS (2014) Optical coherence tomography in Parkinson’s disease: is the retina a biomarker? J Parkinsons Dis 4:197–204. https://doi.org/10.3233/JPD-130306
Tian T, Zhu X-H, Liu Y-H (2011) Potential role of retina as a biomarker for progression of Parkinson’s disease. Int J Ophthalmol 4:433–438. https://doi.org/10.3980/j.issn.2222-3959.2011.04.21
Brandies R, Yehuda S (2008) The possible role of retinal dopaminergic system in visual performance. Neurosci Biobehav Rev 32:611–656. https://doi.org/10.1016/j.neubiorev.2007.09.004
Nguyen-Legros J, Versaux-Botteri C, Vernier P (1999) Dopamine receptor localization in the mammalian retina. Mol Neurobiol 19:181–204. https://doi.org/10.1007/BF02821713
Witkovsky P (2004) Dopamine and retinal function. Doc Ophthalmol 108:17–40. https://doi.org/10.1023/b:doop.0000019487.88486.0a
Frederick JM, Rayborn ME, Laties AM et al (1982) Dopaminergic neurons in the human retina. J Comp Neurol 210:65–79. https://doi.org/10.1002/cne.902100108
Eglen SJ, Raven MA, Tamrazian E, Reese BE (2003) Dopaminergic amacrine cells in the inner nuclear layer and ganglion cell layer comprise a single functional retinal mosaic. J Comp Neurol 466:343–355. https://doi.org/10.1002/cne.10891
Serfozo C, Barta AG, Horvath E et al (2020) Altered visual functions, macular ganglion cell and papillary retinal nerve fiber layer thickness in early-treated adult PKU patients. Mol Genet Metab Rep 25:100649. https://doi.org/10.1016/j.ymgmr.2020.100649
Tantawy AAG, Adly AAM, Hashem NH et al (2020) Ganglion cell complex thinning in young Gaucher patients: relation to prodromal parkinsonian markers. Mov Disord 35:2211–2219. https://doi.org/10.1002/mds.28256
Pascucci T, Giacovazzo G, Andolina D et al (2012) In vivo catecholaminergic metabolism in the medial prefrontal cortex of ENU2 mice: an investigation of the cortical dopamine deficit in phenylketonuria. J Inherit Metab Dis 35:1001–1009. https://doi.org/10.1007/s10545-012-9473-2
Weill Y, Zimran A, Zadok D et al (2020) Macular ganglion cell complex and peripapillary retinal nerve fiber layer thinning in patients with type-1 Gaucher disease. Int J Mol Sci 21. https://doi.org/10.3390/ijms21197027
McNeill A, Roberti G, Lascaratos G et al (2013) Retinal thinning in Gaucher disease patients and carriers: results of a pilot study. Mol Genet Metab 109:221–223. https://doi.org/10.1016/j.ymgme.2013.04.001
Hopf S, Nowak C, Hennermann JB et al (2020) Saccadic reaction time and ocular findings in phenylketonuria. Orphanet J Rare Dis 15:124. https://doi.org/10.1186/s13023-020-01407-7
Hopf S, Pfeiffer N, Liesenfeld M et al (2019) A comprehensive monocentric ophthalmic study with Gaucher disease type 3 patients: vitreoretinal lesions, retinal atrophy and characterization of abnormal saccades. Orphanet J Rare Dis 14:257. https://doi.org/10.1186/s13023-019-1244-9
Hopf S, Liesenfeld M, Schmidtmann I et al (2018) Age dependent normative data of vertical and horizontal reflexive saccades. PLoS ONE 13:e0204008. https://doi.org/10.1371/journal.pone.0204008
van Wegberg AMJ, MacDonald A, Ahring K et al (2017) The complete European guidelines on phenylketonuria: diagnosis and treatment. Orphanet J Rare Dis 12:162. https://doi.org/10.1186/s13023-017-0685-2
Davies EH, Mengel E, Tylki-Szymanska A et al (2011) Four-year follow-up of chronic neuronopathic Gaucher disease in Europeans using a modified severity scoring tool. J Inherit Metab Dis 34:1053–1059. https://doi.org/10.1007/s10545-011-9347-z
Serfozo C, Barta AG, Horvath E et al (2021) Reduced macular thickness and macular vessel density in early-treated adult patients with PKU. Mol Genet Metab Rep 27:100767. https://doi.org/10.1016/j.ymgmr.2021.100767
Nieves-Moreno M, Martínez-de-la-Casa JM, Morales-Fernández L et al (2018) Impacts of age and sex on retinal layer thicknesses measured by spectral domain optical coherence tomography with Spectralis. PLoS ONE 13:e0194169. https://doi.org/10.1371/journal.pone.0194169
Campistol Plana J (2019) Early diagnosis of phenylketonuria. Physiopathology of the neuronal damage and therapeutic options. Medicina (B Aires) 79(Suppl 3):2–5
Cleeter MWJ, Chau K-Y, Gluck C et al (2013) Glucocerebrosidase inhibition causes mitochondrial dysfunction and free radical damage. Neurochem Int 62:1–7. https://doi.org/10.1016/j.neuint.2012.10.010
Klein AD, Mazzulli JR (2018) Is Parkinson’s disease a lysosomal disorder? Brain 141:2255–2262. https://doi.org/10.1093/brain/awy147
Winter AW, Salimi A, Ospina LH, Roos JCP (2019) Ophthalmic manifestations of Gaucher disease: the most common lysosomal storage disorder. Br J Ophthalmol 103:315–326. https://doi.org/10.1136/bjophthalmol-2018-312846
Shrier EM, Barr CC, Grabowski GA (2004) Vitreous opacities and retinal vascular abnormalities in Gaucher disease. Arch Ophthalmol 122:1395–1398. https://doi.org/10.1001/archopht.122.9.1395
Matos A, Gurgel V, Gonçalves M (2017) Ophthalmologic findings in Gaucher’s disease type III: case report. Revista Brasileira de Oftalmologia 76. https://doi.org/10.5935/0034-7280.20170066
La Morgia C, Di Vito L, Carelli V, Carbonelli M (2017) Patterns of retinal ganglion cell damage in neurodegenerative disorders: parvocellular vs magnocellular degeneration in optical coherence tomography studies. Front Neurol 8:710. https://doi.org/10.3389/fneur.2017.00710
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data collection was performed by Susanne Hopf, Julia Hennermann, and Susanne Pitz. Data analysis was performed by Alexander Schuster. Management and interpretation of data were conducted by Susanne Hopf and Alexander Schuster. The first draft of the manuscript was written by Susanne Hopf, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the Medical Ethical Committee of the State Chamber of Medicine of Rhineland Palatinate in Mainz, Germany (reference number 837.373.14). The research adhered to the tenets of the Declaration of Helsinki.
Consent to participate
Informed consent was obtained from all individual participants included in the study, or from legal guardians.
Consent for publication
Participants signed informed consent regarding publishing their data.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hopf, S., Schuster, A.K., Hennermann, J.B. et al. Retinal thinning in phenylketonuria and Gaucher disease type 3. Graefes Arch Clin Exp Ophthalmol 260, 1153–1160 (2022). https://doi.org/10.1007/s00417-021-05424-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-021-05424-5