Abstract
Purpose
Premature birth, race, and sex are contributing risk factors for retinopathy of prematurity (ROP) and have long-term impact on children’s retinal structure. Few studies investigate impact of race and sex on macular structure in children born preterm. This study compared foveal structure in preterm and full-term children.
Methods
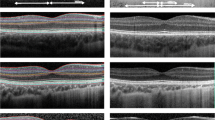
Children aged 4–18 years were enrolled into three groups: (1) ROP-risk group (n = 81), born at < 32 weeks gestational age with and without history of ROP; (2) preterm group (n = 46), born at 32–36 weeks gestational age; and (3) control group (n = 68) with full-term birth. Using spectral-domain optical coherence tomography volume-scan images, foveal structure within 1-mm and 3-mm early treatment diabetic retinopathy study circular grid was measured and segmented. Total inner and outer retina thickness of the right eye was compared among the three groups.
Results
The mean total foveal thickness (in microns) was 287 ± 26 for the ROP-risk group, 276 ± 19 for the preterm group, and 263 ± 20 for the control group (F = 26, p < 0.001). Foveal thickness of the ROP-risk group was significantly higher than that of the preterm group and the control group (all p < 0.05). Foveal thickness was thinner in black children than in white children and thinner in females than in males (all p < 0.001). A similar disparity in race and sex was found in the thickness of the inner and outer layers.
Conclusions
The fovea was significantly thicker in the ROP-risk group than the control group. Foveal thickness decreases with increased gestational age. Race and sex are significant factors in foveal structure in children.




Similar content being viewed by others
Data availability
Available upon request from the corresponding author.
Code availability
Not applicable.
References
Wang J, Spencer R, Leffler JN (2012) Birch EE (2012) Critical period for foveal fine structure in children with regressed retinopathy of prematurity. Retina 32:330–339. https://doi.org/10.1097/IAE.0b013e318219e685
Rosen R, Sjostrand J, Nilsson M, Hellgren K (2015) A methodological approach for evaluation of foveal immaturity after extremely preterm birth. Ophthalmic Physiol Opt 35:433–441. https://doi.org/10.1111/opo.12221
Yanni SE, Wang J, Chan M, Carroll J, Farsiu S, Leffler JN, Spencer R, Birch EE (2012) Foveal avascular zone and foveal pit formation after preterm birth. Br J Ophthalmol 96:961–966. https://doi.org/10.1136/bjophthalmol-2012-301612
Quinn GE, Barr C, Bremer D, Fellows R, Gong A, Hoffman R, Repka MX, Shepard J, Siatkowski RM, Wade K, Ying GS (2016) Changes in course of retinopathy of prematurity from 1986 to 2013: comparison of three studies in the United States. Ophthalmology 123:1595–1600. https://doi.org/10.1016/j.ophtha.2016.03.026
Saunders RA, Donahue ML, Christmann LM, Pakalnis AV, Tung B, Hardy RJ, Phelps DL (1997) Racial variation in retinopathy of prematurity. The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Arch Ophthalmol 115:604–608. https://doi.org/10.1001/archopht.1997.01100150606005
Yang MB, Donovan EF, Wagge JR (2006) Race, gender, and clinical risk index for babies (CRIB) score as predictors of severe retinopathy of prematurity. J AAPOS 10:253–261. https://doi.org/10.1016/j.jaapos.2006.01.004
Asefzadeh B, Cavallerano AA, Fisch BM (2007) Racial differences in macular thickness in healthy eyes. Optom Vis Sci 84:941–945. https://doi.org/10.1097/OPX.0b013e318157a6a0
Kashani AH, Zimmer-Galler IE, Shah SM, Dustin L, Do DV, Eliott D, Haller JA, Dong Nguyen Q (2010) Retinal thickness analysis by race, gender, and age using Stratus OCT. Am J Ophthalmol 149:496-502.e1. https://doi.org/10.1016/j.ajo.2009.09.025
Grover S, Murthy RK, Brar VS, Chalam KV (2009) Normative data for macular thickness by high-definition spectral-domain optical coherence tomography (spectralis). Am J Ophthalmol 148:266–271. https://doi.org/10.1016/j.ajo.2009.03.006
Wagner-Schuman M, Dubis AM, Nordgren RN, Lei Y, Odell D, Chiao H, Weh E, Fischer W, Sulai Y, Dubra A, Carroll J (2011) Racial and gender differences in retinal thickness and foveal pit morphology. Invest Ophthalmol Vis Sci 52:625–634. https://doi.org/10.1167/iovs.10-5886
Kelty PJ, Payne JF, Trivedi RH, Kelty J, Bowie EM, Burger BM (2008) Macular thickness assessment in healthy eyes based on ethnicity using Stratus OCT optical coherence tomography. Invest Ophthalmol Vis Sci 49:2668–2672. https://doi.org/10.1167/iovs.07-1000
Pilat AV, Proudlock FA, Mohammad S, Gottlob I (2014) Normal macular structure measured with optical coherence tomography across ethnicity. Br J Ophthalmol 98:941–945. https://doi.org/10.1136/bjophthalmol-2013-303119
Hsu ST, Ngo HT, Stinnett SS, Cheung NL, House RJ, Kelly MP, Chen X, Enyedi LB, Prakalapakorn SG, Materin MA, El-Dairi MA, Jaffe GJ, Freeman SF, Toth CA, Vajzovic L (2019) Assessment of macular microvasculature in healthy eyes of infants and children using OCT angiography. Ophthalmology 126:1703–1711. https://doi.org/10.1016/j.ophtha.2019.06.028
Hendrickson A, Possin D, Vajzovic L, Toth CA (2012) Histologic development of the human fovea from midgestation to maturity. Am J Ophthalmol 154:767-778.e2. https://doi.org/10.1016/j.ajo.2012.05.007
Hendrickson AE, Yuodelis C (1984) The morphological development of the human fovea. Ophthalmology 91:603–612. https://doi.org/10.1016/s0161-6420(84)34247-6
Duke-Elder S (1963) System ophthalmology. Mosby, St. Louis
Recchia FM, Carvalho-Recchia CA, Trese MT (2002) Optical coherence tomography in the diagnosis of foveal hypoplasia. Arch Ophthalmol 120:1587–1588
Hsu ST, Ngo HT, Stinnett SS, Cheung NL, House RJ, Kelly MP, Chen X, Enyedi LB, Prakalapakorn SG, Materin MA, El-Dairi MA, Jaffe GJ, Freeman SF, Toth CA, Vajzovic L (2019) Assessment of macular microvasculature in healthy eyes in infants and children using OCT angiography. Ophthalmology 126:1703–1711
El-Dairi MA, Asrani SG, Enyedi LB, Freedman SF (2009) Optical coherence tomography in the eyes of normal children. Arch Ophthalmol 127:50–58. https://doi.org/10.1001/archophthalmol.2008.553
Noval S, Freedman SF, Asrani S, El-Dairi MA (2014) Incidence of fovea plana in normal children. J AAPOS 18:471–475. https://doi.org/10.1016/j.jaapos.2014.07.157
Barrio-Barrio J, Noval S, Galdos M, Ruiz-Canela M, Bonet E, Capote M, Lopez M (2013) Multicenter Spanish study of spectral-domain optical coherence tomography in normal children. ActaOphthalmol 91:e56–e63. https://doi.org/10.1111/j.1755-3768.2012.02562.x
Elia N, Pueyo V, Altemir I, Oros D, Pablo LE (2012) Normal reference ranges of optical coherence tomography parameters in childhood. Br J Ophthalmol 96:665–670. https://doi.org/10.1136/bjophthalmol-2011-300916
Moreno TA, O’Connell RV, Chiu SJ, Farsiu S, Cabrera MT, Maldonado RS, Tran-Viet D, Freedman SF, Wallace DK, Toth CA (2013) Choroid development and feasibility of choroidal imaging in the preterm and term infants utilizing SD-OCT. Invest Ophthalmol Vis Sci 54:4140–4147. https://doi.org/10.1167/iovs.12-11471
Odell D, Dubis AM, Lever JF, Stepien KE, Carroll J (2011) Assessing errors inherent in OCT-derived macular thickness maps. J Ophthalmol 2011:692574. https://doi.org/10.1155/2011/692574
Acknowledgements
The authors thank Kim Eissmann for editing the text and thank the reviewers for their invaluable critiques and suggestions to improve the manuscript.
Funding
This work is supported by grants from the National Eye Institute (EY026664), the Pennsylvania Lions Sight Conservation and Eye Research Foundation Grant and by an Institutional Development Award (IDeA) under grant number U54-GM104941 and an award number P20GM13446 from the National Institute of General Medical Sciences of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
Jing Jin made substantial contributions to the conceptualization, methodology, software, validation, formal analysis, investigation, resources, data curation, writing of the original draft, reviewing and editing, visualization, supervision, project administration, and funding acquisition.
Amanda Friess, Dorothy Hendricks, Sharon Lehman, Jonathan Salvin, and Julia E Reid made substantial contributions to data curation, review and editing of the manuscript, and visualization.
Jingyun Wang made substantial contributions to the conceptualization, methodology, software, formal analysis, investigation, data curation, writing of the original draft, reviewing and editing, visualization, and project administration.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Institutional Review Board of the Nemours Office of Human Subject Protection and conformed to the requirements of the United States Health Insurance Portability and Privacy Act.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dr. Amanda Friess and Dr. Jonathan Salvin have left Nemours/Alfred I. duPont Hospital for Children.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jin, J., Friess, A., Hendricks, D. et al. Effect of gestational age at birth, sex, and race on foveal structure in children. Graefes Arch Clin Exp Ophthalmol 259, 3137–3148 (2021). https://doi.org/10.1007/s00417-021-05191-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-021-05191-3