Abstract
Purpose
To prospectively evaluate the effect of benzalkonium chloride (BAK)-preserved latanoprost on ocular surface damage and identify the associated risk factors among treatment-naive glaucoma patients.
Methods
The basal Schirmer’s test results, corneal Oxford staining score, non-invasive keratograph tear-breakup time, oculus hyperemia index score (objective metrics), and ocular surface disease index (OSDI) questionnaire (subjective metric) were evaluated at baseline, 1 month, and 4 months after receiving latanoprost eye drops. Associated risk factors were assessed by multivariate linear regression.
Results
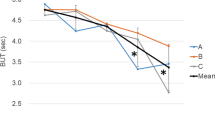
Seventy-four eyes (44 patients) were enrolled. Basal Schirmer’s test tear-flow and Oxford scores gradually deteriorated (β = −0.14, P = 0.001 and β = 0.1, P < 0.001, respectively). The percentage of unstable tear-film (breakup time < 10 s) increased significantly at 4 months (6.21% vs 9.11%, P = 0.042). Hyperemic scores increased significantly at 1 month and normalized at 4 months (P = 0.01 and P = 0.16, respectively); total OSDI scores tended to improve (β = −0.76, P = 0.06). Older age was associated with additional corneal Oxford staining (P = 0.005); female sex was associated with increased unstable tear-film scores (P = 0.01). Artificial tear use was associated with a smaller decrease in basal Schirmer’s test values (P = 0.01) and a smaller increase in unstable tear-film scores (P = 0.02).
Conclusions
Preserved latanoprost eye drops affected ocular surface changes in glaucoma patients through decreased basal tear secretion. Artificial tears represent an early intervention in vulnerable glaucoma patients with reduced tear secretion and impaired tear-film stability.


Similar content being viewed by others
Change history
05 May 2022
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s00417-022-05690-x
References
Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY (2014) Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 121:2081–2090. https://doi.org/10.1016/j.ophtha.2014.05.013
Lowry EA, Chansangpetch S, Lin SC (2019) Use of topical intraocular pressure-lowering medications in the US population: results from the NHANES study 1999 to 2014. J Glaucoma 28:772–776. https://doi.org/10.1097/IJG.0000000000001315
Leung EW, Medeiros FA, Weinreb RN (2008) Prevalence of ocular surface disease in glaucoma patients (2008). J Glaucoma 17:350–355. https://doi.org/10.1097/IJG.0b013e31815c5f4f
Ghosh S, O'Hare F, Lamoureux E, Vajpayee RB, Crowston JG (2012) Prevalence of signs and symptoms of ocular surface disease in individuals treated and not treated with glaucoma medication. Clin Exp Ophthalmol 40:675–681. https://doi.org/10.1111/j.1442-9071.2012.02781.x
Brewitt H, Sistani F (2001) Dry eye disease: the scale of the problem. Surv Ophthalmol 45:S199–S202. https://doi.org/10.1016/s0039-6257(00)00202-2
Methodologies to diagnose and monitor dry eye disease: report of the Diagnostic Methodology Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf 5:108–152. https://doi.org/10.1016/s1542-0124(12)70083-6
Pflugfelder SC, Baudouin C (2011) Challenges in the clinical measurement of ocular surface disease in glaucoma patients. Clin Ophthalmol 5:1575–1583. https://doi.org/10.2147/OPTH.S24410
Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL (2000) Reliability and validity of the ocular surface disease index. Arch Ophthalmol 118:615–621. https://doi.org/10.1001/archopht.118.5.615
Skalicky SE, Goldberg I, McCluskey P (2012) Ocular surface disease and quality of life in patients with glaucoma. Am J Ophthalmol 153 e2:1–9. https://doi.org/10.1016/j.ajo.2011.05.033
Perez-Bartolome F, Martinez-de-la-Casa JM, Arriola-Villalobos P, Fernandez-Perez C, Polo V, Garcia-Feijoo J (2017) Ocular surface disease in patients under topical treatment for glaucoma. Eur J Ophthalmol 27:694–704. https://doi.org/10.5301/ejo.5000977
Fechtner RD, Godfrey DG, Budenz D, Stewart JA, Stewart WC, Jasek MC (2010) Prevalence of ocular surface complaints in patients with glaucoma using topical intraocular pressure-lowering medications. Cornea 29:618–621. https://doi.org/10.1097/ICO.0b013e3181c325b2
Mathews PM, Ramulu PY, Friedman DS, Utine CA, Akpek EK (2013) Evaluation of ocular surface disease in patients with glaucoma. Ophthalmology 120:2241–2248. https://doi.org/10.1016/j.ophtha.2013.03.045
van Bijsterveld OP (1969) Diagnostic tests in the sicca syndrome. Arch Ophthalmol 82:10–14. https://doi.org/10.1001/archopht.1969.00990020012003
Mengher LS, Pandher KS, Bron AJ (1986) Non-invasive tear film break-up time: sensitivity and specificity. Acta Ophthalmol 64:441–444. https://doi.org/10.1111/j.1755-3768.1986.tb06950.x
Jiang Y, Ye H, Xu J, Lu Y (2014) Noninvasive Keratograph assessment of tear film break-up time and location in patients with age-related cataracts and dry eye syndrome. J Int Med Res 42:494–502. https://doi.org/10.1177/0300060513504701
Cvenkel B, Štunf S, Srebotnik Kirbiš I, Strojan Fležar M (2015) Symptoms and signs of ocular surface disease related to topical medication in patients with glaucoma. Clin Ophthalmol 9:625–631. https://doi.org/10.2147/OPTH.S81247
Baudouin C, Hamard P, Liang H, Creuzot-Garcher C, Bensoussan L, Brignole F (2004) Conjunctival epithelial cell expression of interleukins and inflammatory markers in glaucoma patients treated over the long term. Ophthalmology 111:2186–2192. https://doi.org/10.1016/j.ophtha.2004.06.023
Sarkar J, Chaudhary S, Namavari A, Ozturk O, Chang JH, Yco L, Sonawane S, Khanolkar V, Hallak J, Jain S (2012) Corneal neurotoxicity due to topical benzalkonium chloride. Invest Ophthalmol Vis Sci 53:1792–1802. https://doi.org/10.1167/iovs.11-8775
Pisella PJ, Pouliquen P, Baudouin C (2002) Prevalence of ocular symptoms and signs with preserved and preservative free glaucoma medication. Br J Ophthalmol 86:418–423. https://doi.org/10.1136/bjo.86.4.418
Ramli N, Supramaniam G, Samsudin A, Juana A, Zahari M, Choo MM (2015) Ocular surface disease in Glaucoma: effect of polypharmacy and preservatives. Optom Vis Sci 92:e222–e226. https://doi.org/10.1097/OPX.0000000000000542
Wu S, Hong J, Tian L, Cui X, Sun X, Xu J (2015) Assessment of bulbar redness with a newly developed Keratograph. Optom Vis Sci 92:892–899. https://doi.org/10.1097/OPX.0000000000000643
Bron AJ, Evans VE, Smith JA (2003) Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea 22:640–650. https://doi.org/10.1097/00003226-200310000-00008
Pauly A, Roubeix C, Liang H, Brignole-Baudouin F, Baudouin C (2012) In vitro and in vivo comparative toxicological study of a new preservative-free latanoprost formulation. Invest Ophthalmol Vis Sci 53:8172–8180. https://doi.org/10.1167/iovs.12-10766
Liang H, Baudouin C, Pauly A, Brignole-Baudouin F (2008) Conjunctival and corneal reactions in rabbits following short- and repeated exposure to preservative-free tafluprost, commercially available latanoprost and 0.02% benzalkonium chloride. Br J Ophthalmol 92:1275–1582. https://doi.org/10.1136/bjo.2008.138768
Benitez del Castillo JM, Wasfy MA, Fernandez C, Garcia-Sanchez J (2004) An in vivo confocal masked study on corneal epithelium and subbasal nerves in patients with dry eye. Invest Ophthalmol Vis Sci 45:3030–3035. https://doi.org/10.1167/iovs.04-0251
Cruzat A, Qazi Y, Hamrah P (2017) In vivo confocal microscopy of corneal nerves in health and disease. Ocul Surf 15:15–47. https://doi.org/10.1016/j.jtos.2016.09.004
Portela RC, Fares NT, Machado LF, São Leão AF, de Freitas D, Paranhos A Jr, Prata TS, Gracitelli CPB (2008) Evaluation of ocular surface disease in patients with Glaucoma: clinical parameters, self-report assessment and Keratograph analysis. J Glaucoma 27:794–801. https://doi.org/10.1097/IJG.0000000000001007
Truong S, Cole N, Stapleton F, Golebiowski B (2014) Sex hormones and the dry eye. Clin Exp Optom 97:324–336. https://doi.org/10.1111/cxo.12147
Malik A, Claoue C (2012) Transport and interaction of cosmetic product material within the ocular surface: beauty and the beastly symptoms of toxic tears. Cont Lens Anterior Eye 35:247–259. https://doi.org/10.1016/j.clae.2012.07.005
Arcieri ES, Santana A, Rocha FN, Guapo GL, Costa VP (2005) Blood-aqueous barrier changes after the use of prostaglandin analogues in patients with pseudophakia and aphakia: a 6-month randomized trial. Arch Ophthalmol 123:186–192. https://doi.org/10.1001/archopht.123.2.186
Alm A (1998) Prostaglandin derivates as ocular hypotensive agents. Prog Retin Eye Res 17:291–312. https://doi.org/10.1016/s1350-9462(97)00003-7
Markoulli M, Sobbizadeh A, Tan J, Briggs N, Coroneo M (2018) The effect of optive and optive advanced artificial tears on the healthy tear film. Curr Eye Res 43:588–594. https://doi.org/10.1080/02713683.2018.1433860
Lanzini M, Curcio C, Colabelli-Gisoldi RA, Mastropasqua A, Calienno R, Agnifili L, Nubile M, Mastropasqua L (2015) In vivo and impression cytology study on the effect of compatible solutes eye drops on the ocular surface epithelial cell quality in dry eye patients. Mediat Inflamm 351424:2015. https://doi.org/10.1155/2015/351424
Calvao-Santos G, Borges C, Nunes S, Salgado-Borges J, Duarte L (2011) Efficacy of 3 different artificial tears for the treatment of dry eye in frequent computer users and/or contact lens users. Eur J Ophthalmol 21:538–544. https://doi.org/10.5301/EJO.2011.6324
Acknowledgements
The authors acknowledge statistical assistance provided by the Center of Statistical Consultation and Research in the Department of Medical Research, National Taiwan University Hospital.
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
All procedures used in this study were approved by the Ethics Review Board of the National Taiwan University Hospital and adhered to the tenets of the Declaration of Helsinki.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail:https://doi.org/10.1007/s00417-022-05690-x
About this article
Cite this article
Su, CC., Lee, YC. & Lee, P.R.C. RETRACTED ARTICLE: Assessment of ocular surface disease in glaucoma patients with benzalkonium chloride-preserved latanoprost eye drops: a short-term longitudinal study. Graefes Arch Clin Exp Ophthalmol 259, 1243–1251 (2021). https://doi.org/10.1007/s00417-020-05067-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-020-05067-y