Abstract
Purpose
To develop a deep learning (DL) model for automated detection of glaucoma and to compare diagnostic capability against hand-craft features (HCFs) based on spectral domain optical coherence tomography (SD-OCT) peripapillary retinal nerve fiber layer (pRNFL) images.
Methods
A DL model with pre-trained convolutional neural network (CNN) based was trained using a retrospective training set of 1501 pRNFL OCT images, which included 690 images from 153 glaucoma patients and 811 images from 394 normal subjects. The DL model was further tested in an independent test set of 50 images from 50 glaucoma patients and 52 images from 52 normal subjects. A customized software was used to extract and measure HCFs including pRNFL thickness in average and four different sectors. Area under the receiver operator characteristics (AROC) curves was calculated to compare the diagnostic capability between DL model and hand-crafted pRNFL parameters.
Results
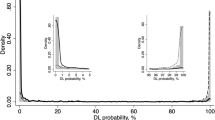
In this study, the DL model achieved an AROC of 0.99 [CI: 0.97 to 1.00] which was significantly larger than the AROC values of all other HCFs (AROCs 0.661 with 95% CI 0.549 to 0.772 for temporal sector, AROCs 0.696 with 95% CI 0.549 to 0.799 for nasal sector, AROCs 0.913 with 95% CI 0.855 to 0.970 for superior sector, AROCs 0.938 with 95% CI 0.894 to 0.982 for inferior sector, and AROCs 0.895 with 95% CI 0.832 to 0.957 for average).
Conclusion
Our study demonstrated that DL models based on pre-trained CNN are capable of identifying glaucoma with high sensitivity and specificity based on SD-OCT pRNFL images.




Similar content being viewed by others
References
Quigley HA (2011) Glaucoma. Lancet 377:1367–1377
Weinreb RN, Aung T, Medeiros FA (2014) The pathophysiology and treatment of glaucoma: a review. JAMA 311:1901–1911
Sung KR, Kim JS, Wollstein G et al (2011) Imaging of the retinal nerve fibre layer with spectral domain optical coherence tomography for glaucoma diagnosis. Br J Ophthalmol 95:909–914. https://doi.org/10.1136/bjo.2010.186924
Medeiros FA, Zangwill LM, Bowd C et al (2005) Evaluation of retinal nerve fiber layer, optic nerve head, and macular thickness measurements for glaucoma detection using optical coherence tomography. Am J Ophthalmol 139:44–55
Leung CK, Cheung CY, Weinreb RN et al (2009) Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: a variability and diagnostic performance study. Ophthalmology 116:1257–1263
Knight OJ, Chang RT, Feuer WJ et al (2009) Comparison of retinal nerve fiber layer measurements using time domain and spectral domain optical coherent tomography. Ophthalmology 116:1271–1277
Asaoka R, Murata H, Hirasawa K et al (2018) Using deep learning and transform learning to accurately diagnose early-onset glaucoma from macular optical coherence tomography images. Am J Ophthalmol. https://doi.org/10.1016/j.ajo.2018.10.007 (1879-1891 (Electronic))
Gulshan V, Peng L, Coram M et al (2016) Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA 316(22):2402
Shen W, Zhou M, Yang F et al (2015) Multi-scale convolutional neural networks for lung nodule classification. Info Process Med Imaging 24:588–599
Lakhani P, Sundaram B (2017) Deep learning at chest radiography: automated classification of pulmonary tuberculosis by using convolutional neural networks. Radiology 284(2):574–582. https://doi.org/10.1148/radiol.2017162326
Le MH, Chen J, Wang L et al (2017) Automated diagnosis of prostate cancer in multi-parametric MRI based on multimodal convolutional neural networks. Phys Med Biol 62(16):6491–6514. https://doi.org/10.1088/1361-6560/aa7731
Asaoka R, Murata H, Iwase A et al (2016) Detecting preperimetric glaucoma with standard automated perimetry using a deep learning classifier. Ophthalmology 123(9):1974–1980. https://doi.org/10.1016/j.ophtha.2016.05.029
Rasband WS. ImageJ, US. National Institutes of Health, Bethesda, Maryland, USA. http://imagej.nih.gov/ij/. Accessed 2 Oct 2019
Shabana N, Aquino MC, See J et al (2012) Quantitative evaluation of anterior chamber parameters using anterior segment optical coherence tomography in primary angle closure mechanisms. Clin Exp Ophthalmol 40(8):792–801. https://doi.org/10.1111/j.1442-9071.2012.02805.x
Zheng C, de Leon JM, Cheung CY et al (2016) Determinants of pupil diameters and pupil dynamics in an adult Chinese population. Graefes Arch Clin Exp Ophthalmol 254(5):929–936. https://doi.org/10.1007/s00417-016-3272-7
Chen B, Gao E, Chen H et al (2016) Profile and determinants of retinal optical intensity in normal eyes with spectral domain optical coherence tomography. PLoS One 11(2):1–16. https://doi.org/10.1371/journal.pone.0148183
Russakovsky O, Deng J, Su H et al (2015) ImageNet large scale visual recognition challenge. Int J Comput Vis 115.3(2015):211–252. https://doi.org/10.1007/s11263-015-0816-y
Szegedy C, Vanhoucke V, Ioffe S, et al. (2016) Rethinking the inception architecture for computer vision. 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR). 2818-2826.doi:https://doi.org/10.1109/CVPR.2016.308
Zhou B, Khosla A, Lapedriza A, et al. (2015) Learning deep features for discriminative localization. CVPR'16 (arXiv:1512.04150, 2015)
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44(3):837–845
Rao HL, Zangwill LM, Weinreb RN et al (2010) Comparison of different spectral domain optical coherence tomography scanning areas for glaucoma diagnosis. Ophthalmology 117(9):1692–1699.e1. https://doi.org/10.1016/j.ophtha.2010.01.031
Seong M, Sung KR, Choi EH et al (2010) Macular and peripapillary retinal nerve fiber layer measurements by spectral domain optical coherence tomography in normal-tension glaucoma. Investig Ophthalmol Vis Sci 51(3):1446–1452. https://doi.org/10.1167/iovs.09-4258
Wang X, Peng Y, Lu L, et al. (2017) ChestX-ray8: Hospital-scale chest x-ray database and benchmarks on weakly-supervised classification and localization of common thorax diseases. 2017 IEEE Conference on Computer Vision and Pattern Recognition (CVPR). 3462-3471. https://doi.org/10.1109/CVPR.2017.369
Yosinski J, Clune J, Nguyen A, et al. (2015) Understanding neural networks through deep visualization. arXiv preprint. https://arxiv.org/abs/1506.06579. Published June 22, 2015. Accessed Augest 12, 2019
Vermeer KA, van der Schoot J, Lemij HG et al (2012) RPE-normalized RNFL attenuation coefficient maps derived from volumetric OCT imaging for glaucoma assessment. Investig Ophthalmol Vis Sci 53(10):6102–6108. https://doi.org/10.1167/iovs.12-9933
Xu H, Zhai R, Zong Y et al (2018) Comparison of retinal microvascular changes in eyes with high-tension glaucoma or normal-tension glaucoma: a quantitative optic coherence tomography angiographic study. Graefes Arch Clin Exp Ophthcalmol 256(6):1179–1186
Rolle T, Briamonte C, Curto D et al (2011) Ganglion cell complex and retinal nerve fiber layer measured by fourier-domain optical coherence tomography for early detection of structural damage in patients with preperimetric glaucoma. Clin Ophthalmol 5:961–969. https://doi.org/10.2147/OPTH.S20249
Kim S Y , Park H Y L , Park C K . (2012) The effects of peripapillary atrophy on the diagnostic ability of stratus and cirrus oct in the analysis of optic nerve head parameters and disc size [J]. Invest Ophthalmol Vis Sci 53(8)
Funding
This study was funded by the National Natural Science Foundation of China (81371010) and Clinical Research Funds of Shantou University Medical College (2014).
Author information
Authors and Affiliations
Contributions
CZ, MZZ, and ZF contributed towards the conception and design, drafted the manuscript, and approved the final version. CZ, JWL, and LTH developed the artificial intelligence system evaluated in this study. XLX, JLY, TQ and BYC gathered, cleaned, and organized the data.
Corresponding author
Ethics declarations
This study was conducted according to the tenets of the Declaration of Helsinki and had the approval of the institutional review board.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zheng, C., Xie, X., Huang, L. et al. Detecting glaucoma based on spectral domain optical coherence tomography imaging of peripapillary retinal nerve fiber layer: a comparison study between hand-crafted features and deep learning model. Graefes Arch Clin Exp Ophthalmol 258, 577–585 (2020). https://doi.org/10.1007/s00417-019-04543-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-019-04543-4