Abstract
Purpose
Intravitreal injections with anti-vascular endothelial growth factor (anti-VEGF) medications have become the standard of care for their respective indications. Optical coherence tomography (OCT) scans of the central retina provide detailed anatomical data and are widely used by clinicians in the decision-making process of anti-VEGF indication. In recent years, significant progress has been made in artificial intelligence and computer vision research. We trained a deep convolutional artificial neural network to predict treatment indication based on central retinal OCT scans without human intervention.
Method
A total of 183,402 retinal OCT B-scans acquired between 2008 and 2016 were exported from the institutional image archive of a university hospital. OCT images were cross-referenced with the electronic institutional intravitreal injection records. OCT images with a following intravitreal injection during the first 21 days after image acquisition were assigned into the ‘injection’ group, while the same amount of random OCT images without intravitreal injections was labeled as ‘no injection’. After image preprocessing, OCT images were split in a 9:1 ratio to training and test datasets. We trained a GoogLeNet inception deep convolutional neural network and assessed its performance on the validation dataset. We calculated prediction accuracy, sensitivity, specificity, and receiver operating characteristics.
Results
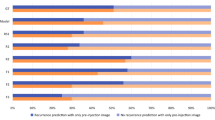
The deep convolutional neural network was successfully trained on the extracted clinical data. The trained neural network classifier reached a prediction accuracy of 95.5% on the images in the validation dataset. For single retinal B-scans in the validation dataset, a sensitivity of 90.1% and a specificity of 96.2% were achieved. The area under the receiver operating characteristic curve was 0.968 on a per B-scan image basis, and 0.988 by averaging over six B-scans per examination on the validation dataset.
Conclusion
Deep artificial neural networks show impressive performance on classification of retinal OCT scans. After training on historical clinical data, machine learning methods can offer the clinician support in the decision-making process. Care should be taken not to mistake neural network output as treatment recommendation and to ensure a final thorough evaluation by the treating physician.



Similar content being viewed by others
References
Parikh R, Ross JS, Sangaralingham LR et al (2017) Trends of anti-vascular endothelial growth factor use in ophthalmology among privately insured and Medicare advantage patients. Ophthalmology 124:352–358. https://doi.org/10.1016/j.ophtha.2016.10.036
Keane PA, Patel PJ, Liakopoulos S et al (2012) Evaluation of age-related macular degeneration with optical coherence tomography. Surv Ophthalmol 57:389–414. https://doi.org/10.1016/j.survophthal.2012.01.006
Huang D, Swanson EA, Lin CP et al (1991) Optical coherence tomography. Science 254:1178–1181
Drexler W, Fujimoto JG (2008) State-of-the-art retinal optical coherence tomography. Prog Retin Eye Res 27:45–88. https://doi.org/10.1016/j.preteyeres.2007.07.005
Patel PJ, Browning AC, Chen FK et al (2009) Interobserver agreement for the detection of optical coherence tomography features of neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci 50:5405–5410. https://doi.org/10.1167/iovs.09-3505
Framme C, Panagakis G, Walter A et al (2012) Interobserver variability for retreatment indications after ranibizumab loading doses in neovascular age-related macular degeneration. Acta Ophthalmol 90:49–55. https://doi.org/10.1111/j.1755-3768.2010.01940.x
LeCun Y, Bengio Y, Hinton G (2015) Deep learning. Nature 521:436–444. https://doi.org/10.1038/nature14539
Simonyan K, Zisserman A (2014) Very deep convolutional networks for large-scale image recognition. http://arxiv.org/abs/1409.1556v6 . Accessed 12 Feb 2017
Krizhevsky A, Sutskever I, Hinton GE (2012) ImageNet classification with deep convolutional neural networks. In: Pereira F, Burges CJC, Bottou L, Weinberger KQ (eds) Advances in Neural Information Processing Systems 25 (NIPS 2012). MIT Press, Cambridge MA, pp 1097–1105
Szegedy C, Liu W, Jia Y, et al. (2014) Going deeper with convolutions. http://arxiv.org/abs/1409.4842v1 . Accessed 12 Feb 2017
Abràmoff MD, Lou Y, Erginay A et al (2016) Improved automated detection of diabetic retinopathy on a publicly available dataset through integration of deep learning. Invest Ophthalmol Vis Sci 57:5200–5206. https://doi.org/10.1167/iovs.16-19964
Asaoka R, Murata H, Iwase A, Araie M (2016) Detecting preperimetric glaucoma with standard automated perimetry using a deep learning classifier. Ophthalmology 123:1974–1980. https://doi.org/10.1016/j.ophtha.2016.05.029
Lee CS, Baughman DM, Lee AY (2016) Deep learning is effective for the classification of OCT images of normal versus age-related macular degeneration. http://arxiv.org/abs/1612.04891v1 . Accessed 12 Feb 2017
Ioffe S, Szegedy C (2015) Batch normalization: accelerating deep network training by reducing internal covariate shift. http://arxiv.org/abs/1502.03167v3 . Accessed 12 Feb 2017
Heng LZ, Pefkianaki M, Pefianaki M et al (2015) Interobserver agreement in detecting spectral-domain optical coherence tomography features of diabetic macular edema. PLoS One 10:e0126557. https://doi.org/10.1371/journal.pone.0126557
Engelbert M, Zweifel SA, Freund KB (2009) ‘Treat and extend’ dosing of intravitreal antivascular endothelial growth factor therapy for type 3 neovascularization/retinal angiomatous proliferation. Retina 29:1424–1431. https://doi.org/10.1097/IAE.0b013e3181bfbd46
Nguyen A, Yosinski J, Clune J (2014) Deep neural networks are easily fooled: high confidence predictions for unrecognizable images. http://arxiv.org/abs/1412.1897v4 . Accessed 12 Mar 2017
Funding
No funding was received for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments. For this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
Prahs, P., Radeck, V., Mayer, C. et al. OCT-based deep learning algorithm for the evaluation of treatment indication with anti-vascular endothelial growth factor medications. Graefes Arch Clin Exp Ophthalmol 256, 91–98 (2018). https://doi.org/10.1007/s00417-017-3839-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-017-3839-y