Abstract
Purpose
To introduce a human cell culture technique for investigating in-vitro behavior of primary epiretinal cells and membrane contraction of fibrocellular tissue surgically removed from eyes with idiopathic macular pucker.
Methods
Human epiretinal membranes were harvested from ten eyes with idiopathic macular pucker during standard vitrectomy. Specimens were fixed on cell culture plastic using small entomological pins to apply horizontal stress to the tissue, and then transferred to standard cell culture conditions. Cell behavior of 400 epiretinal cells from 10 epiretinal membranes was observed in time-lapse microscopy and analyzed in terms of cell migration, cell velocity, and membrane contraction. Immunocytochemistry was performed for cell type-specific antigens.
Results
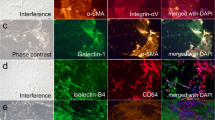
Cell specific differences in migration behavior were observed comprising two phenotypes: (PT1) epiretinal cells moving fast, less directly, with small round phenotype and (PT2) epiretinal cells moving slowly, directly, with elongated large phenotype. No mitosis, no outgrowth and no migration onto the plastic were seen. Horizontal contraction measurements showed variation between specimens. Masses of epiretinal cells with a myofibroblast-like phenotype expressed cytoplasmatic α-SMA stress fibers and correlated with cell behavior characteristics (PT2). Fast moving epiretinal cells (PT1) were identified as microglia by immunostaining.
Conclusions
This in-vitro technique using traction application allows for culturing surgically removed epiretinal membranes from eyes with idiopathic macular pucker, demonstrating cell behavior and membrane contraction of primary human epiretinal cells. Our findings emphasize the abundance of myofibroblasts, the presence of microglia and specific differences of cell behavior in these membranes. This technique has the potential to improve the understanding of pathologies at the vitreomacular interface and might be helpful in establishing anti-fibrotic treatment strategies.






Similar content being viewed by others
References
Mitchell P, Smith W, Chey T, Wang JJ, Chang A (1997) Prevalence and associations of epiretinal membranes. The Blue Mountains eye study, Australia. Ophthalmology 104:1033–1040
Sebag J (2004) Anomalous posterior vitreous detachment: a unifying concept in vitreo-retinal disease. Graefes Arch Clin Exp Ophthalmol 242:690–698. doi:10.1007/s00417-004-0980-1
Schumann RG, Gandorfer A, Ziada J, Scheler R, Schaumberger MM, Wolf A, Kampik A, Haritoglou C (2014) Hyalocytes in idiopathic epiretinal membranes: a correlative light and electron microscopic study. Graefes Arch Clin Exp Ophthalmol 252:1887–1894. doi:10.1007/s00417-014-2841-x
Hiscott PS, Grierson I, Hitchins CA, Rahi AH, McLeod D (1983) Epiretinal membranes in vitro. Trans Ophthalmol Soc U K 103(Pt 1):89–102
Andjelic S, Lumi X, Vereb Z, Josifovska N, Facsko A, Hawlina M, Petrovski G (2014) A simple method for establishing adherent ex vivo explant cultures from human eye pathologies for use in subsequent calcium imaging and inflammatory studies. J Immunol Res 2014:232659. doi:10.1155/2014/232659
Vereb Z, Lumi X, Andjelic S, Globocnik-Petrovic M, Urbancic M, Hawlina M, Facsko A, Petrovski G (2013) Functional and molecular characterization of ex vivo cultured epiretinal membrane cells from human proliferative diabetic retinopathy. Biomed Res Int 2013:492376. doi:10.1155/2013/492376
Ioachim E, Stefaniotou M, Gorezis S, Tsanou E, Psilas K, Agnantis NJ (2005) Immunohistochemical study of extracellular matrix components in epiretinal membranes of vitreoproliferative retinopathy and proliferative diabetic retinopathy. Eur J Ophthalmol 15:384–391
Mori K, Gehlbach PL, Sano A, Deguchi T, Yoneya S (2004) Comparison of epiretinal membranes of differing pathogenesis using optical coherence tomography. Retina 24:57–62
Wormstone IM (2002) Posterior capsule opacification: a cell biological perspective. Exp Eye Res 74:337–347. doi:10.1006/exer.2001.1153
Wertheimer C, Siedlecki J, Kook D, Mayer WJ, Wolf A, Klingenstein A, Kampik A, Eibl-Lindner K (2015) EGFR inhibitor Gefitinib attenuates posterior capsule opacification in vitro and in the ex vivo human capsular bag model. Graefes Arch Clin Exp Ophthalmol 253:409–417. doi:10.1007/s00417-014-2875-0
Wertheimer C, Liegl R, Kernt M, Docheva D, Kampik A, Eibl-Lindner KH (2014) EGFR-blockade with erlotinib reduces EGF and TGF-beta2 expression and the actin-cytoskeleton which influences different aspects of cellular migration in lens epithelial cells. Curr Eye Res 39:1000–1012. doi:10.3109/02713683.2014.888453
Popov C, Kohler J, Docheva D (2015) Activation of EphA4 and EphB2 reverse signaling restores the age-associated reduction of self-renewal, migration, and actin turnover in human tendon stem/progenitor cells. Front Aging Neurosci 7:246. doi:10.3389/fnagi.2015.00246
Collins TJ (2007) ImageJ for microscopy. BioTechniques 43:25–30
Meijering E, Dzyubachyk O, Smal I (2012) Methods for cell and particle tracking. Methods Enzymol 504:183–200. doi:10.1016/B978-0-12-391857-4.00009-4
Benhamou S (2004) How to reliably estimate the tortuosity of an animal’s path: straightness, sinuosity, or fractal dimension? J Theor Biol 229:209–220. doi:10.1016/j.jtbi.2004.03.016
Mrass P, Takano H, Ng LG, Daxini S, Lasaro MO, Iparraguirre A, Cavanagh LL, von Andrian UH, Ertl HC, Haydon PG, Weninger W (2006) Random migration precedes stable target cell interactions of tumor-infiltrating T cells. J Exp Med 203:2749–2761. doi:10.1084/jem.20060710
Beltman JB, Maree AF, de Boer RJ (2009) Analysing immune cell migration. Nat Rev Immunol 9:789–798. doi:10.1038/nri2638
Beltman JB, Maree AF, Lynch JN, Miller MJ, de Boer RJ (2007) Lymph node topology dictates T cell migration behavior. J Exp Med 204:771–780. doi:10.1084/jem.20061278
Zhao F, Gandorfer A, Haritoglou C, Scheler R, Schaumberger MM, Kampik A, Schumann RG (2013) Epiretinal cell proliferation in macular pucker and vitreomacular traction syndrome: analysis of flat-mounted internal limiting membrane specimens. Retina 33:77–88. doi:10.1097/IAE.0b013e3182602087
Wertheimer C, Brandlhuber U, Kook D, Mayer WJ, Laubichler P, Wolf A, Kampik A, Eibl-Lindner K (2015) Erufosine, a phosphoinositide-3-kinase inhibitor, to mitigate posterior capsule opacification in the human capsular bag model. J Cataract Refract Surg 41:1484–1489. doi:10.1016/j.jcrs.2015.02.034
Wertheimer C, Kreutzer TC, Dirisamer M, Eibl-Lindner K, Kook D, Priglinger S, Mayer WJ (2016) Effect of femtosecond laser-assisted lens surgery on posterior capsule opacification in the human capsular bag in vitro. Acta Ophthalmol. doi:10.1111/aos.13103
Taylor L, Moran D, Arnér K, Warrant E, Ghosh F (2013) Stretch to see: lateral tension strongly determines cell survival in long-term cultures of adult porcine retina. Invest Ophthalmol Vis Sci 54(3):1845–1856. doi:10.1167/iovs.12-11420
Abu El-Asrar AM, De Hertogh G, van den Eynde K, Alam K, Van Raemdonck K, Opdenakker G, Van Damme J, Geboes K, Struyf S (2015) Myofibroblasts in proliferative diabetic retinopathy can originate from infiltrating fibrocytes and through endothelial-to-mesenchymal transition (EndoMT). Exp Eye Res 132:179–189. doi:10.1016/j.exer.2015.01.023
Bochaton-Piallat ML, Gabbiani G, Hinz B (2016) The myofibroblast in wound healing and fibrosis: answered and unanswered questions. F1000Res 5. doi:10.12688/f1000research.8190.1
Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C (2001) Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell 12:2730–2741
Klingberg F, Chow ML, Koehler A, Boo S, Buscemi L, Quinn TM, Costell M, Alman BA, Genot E, Hinz B (2014) Prestress in the extracellular matrix sensitizes latent TGF-beta1 for activation. J Cell Biol 207:283–297. doi:10.1083/jcb.201402006
Liu CS, Wormstone IM, Duncan G, Marcantonio JM, Webb SF, Davies PD (1996) A study of human lens cell growth in vitro. A model for posterior capsule opacification. Invest Ophthalmol Vis Sci 37:906–914
Rieu JP, Upadhyaya A, Glazier JA, Ouchi NB, Sawada Y (2000) Diffusion and deformations of single hydra cells in cellular aggregates. Biophys J 79:1903–1914. doi:10.1016/S0006-3495(00)76440-X
Bu SC, Kuijer R, van der Worp RJ, Postma G, Renardel de Lavalette VW, Li XR, Hooymans JM, Los LI (2015) Immunohistochemical evaluation of idiopathic epiretinal membranes and in vitro studies on the effect of TGF-beta on Muller cells. Invest Ophthalmol Vis Sci 56:6506–6514. doi:10.1167/iovs.14-15971
Salzmann J, Limb GA, Khaw PT, Gregor ZJ, Webster L, Chignell AH, Charteris DG (2000) Matrix metalloproteinases and their natural inhibitors in fibrovascular membranes of proliferative diabetic retinopathy. Br J Ophthalmol 84(10):1091–1096. doi:10.1136/bjo.84.10.1091
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Funding
No funding was received for this research.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Financial interest
The authors have no proprietary interest in this study.
Electronic supplementary material
Movie 1
Time-lapse microscopy of idiopathic epiretinal membrane pinned on cell culture plastic with tangential traction application. Differences in cell migration behavior were observed: (1) epiretinal cells moving fast, less directly, with small round phenotype, and (2) epiretinal cells moving slowly, directly, with elongated large phenotype. (MOV 1662 kb)
Movie 2
Time-lapse microscopy of idiopathic epiretinal membrane pinned on cell culture plastic demonstrating membrane contraction. The membrane edge was marked with a reference marker. Based on 10 measurements per specimen, mean drift of the membrane edges towards the centre was 3.1 ± 0.96 μm within 15 h. (MOV 1430 kb)
Rights and permissions
About this article
Cite this article
Wertheimer, C., Eibl-Lindner, K.H., Compera, D. et al. A cell culture technique for human epiretinal membranes to describe cell behavior and membrane contraction in vitro. Graefes Arch Clin Exp Ophthalmol 255, 2147–2155 (2017). https://doi.org/10.1007/s00417-017-3767-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-017-3767-x