Abstract
Purpose
To differentiate the bioelectrical cortical responses driven by axons from central and mid-peripheral retina in Leber’s hereditary optic neuropathy (LHON) by using multifocal visual evoked potentials (mfVEP).
Methods
Seventeen genetically confirmed LHON patients (33.35 ± 8.4 years, 17 eyes) and 22 age-matched controls (C) (38.2 ± 6.0 years, 22 eyes) were studied by mfVEP and optical coherence tomography. MfVEP P1 implicit time (P1 IT, ms) and response amplitude density of the N1-P1 components (N1-P1 RAD, nV/deg2) of the second order binary kernel were measured for five concentric retinal areas between the fovea and mid-periphery: 0–20 degrees (R1 to R5).
Results
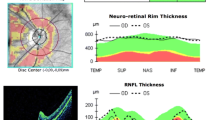
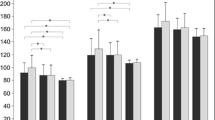
Mean mfVEP P1 ITs and N1-P1 RADs at all five foveal eccentricities were significantly different (p < 0.01) in LHON when compared to controls. In both groups, mean mfVEP responses obtained from R1 to R5 showed a progressive shortening of P1 ITs (linear fitting, LHON: r = −0.95; C: r = −0.98) and decrease of N1-P1 RADs (exponential fitting, LHON: r 2 = 0.94; C: r 2 = 0.93). The slope of the linear fitting between mean mfVEP P1 ITs in the two groups was about three times greater in LHON than in controls (LHON: y = −13.33x +182.03; C: y = −4.528x +108.1). MfVEP P1 ITs detected in R1 and R2 (0–5 degrees) were significantly correlated (p < 0.01) with the reduction of retinal nerve fiber layer thickness of the temporal quadrant.
Conclusions
MfVEP identifies abnormal neural conduction along the visual pathways in LHON, discriminating a predominant involvement of axons driving responses from the central retina when compared to those serving the mid-peripheral retina.



Similar content being viewed by others
References
Young B, Eggenberger E, Kaufman D (2012) Current electrophysiology in ophthalmology: a review. Curr Opin Ophthalmol 23:497–505
Hood DC, Greenstein VC (2003) Multifocal VEP and ganglion cell damage: applications and limitations for the study of glaucoma. Prog Retin Eye Res 22:201–251
Klistorner AI, Graham SL, Grigg JR, Billson FA (1998) Multifocal topographic visual evoked potential: improving objective detection of local visual field defects. Invest Ophthalmol Vis Sci 39:937–950
Parisi V, Ziccardi L, Stifano G, Montrone L, Gallinaro G, Falsini B (2010) Impact of regional retinal responses on cortical visually evoked responses: multifocal ERGs and VEPs in the retinitis pigmentosa model. Clin Neurophysiol 121:380–385
Baseler HA, Sutter EE, Klein SA, Carney T (1994) The topography of visual evoked response properties across the visual field. Electroencephalogr Clin Neurophysiol 90:65–81
Baseler HA, Sutter EE (1997) M and P components of the VEP and their visual field distribution. Vision Res 37:675–690
Park S, Park SH, Chang JH, Ohn YH (2011) Study for analysis of the multifocal visual evoked potential. Korean J Ophthalmol 25:334–340
Carelli V, Barboni P, Sadun AA (2006) Mitochondrial ophthalmology. In: DiMauro S, Hirano M, Shon EA (eds) Mitochondrial medicine. Informa Healthcare, London, pp 105–142
Carelli V, Ross-Cisneros FN, Sadun AA (2004) Mitochondrial dysfunction as a cause of optic neuropathies. Prog Retin Eye Res 23:53–89
Nikoskelainen EK (1994) Clinical picture of LHON. Clin Neurosci 2:115–120
Curcio CA, Allen KA (1990) Topography of ganglion cells in human retina. J Comp Neurol 300:5–25
Wassle H, Grunert U, Rohrenbeck J, Boycott BB (1990) Retinal ganglion cell density and cortical magnification factor in the primate. Vision Res 30:1897–1911
Sutter EE, Bearse MA (1999) The optic nerve head component of the human ERG. Vision Res 39:419–436
Fukuda Y, Watanabe M, Wakakuwa K, Sawai H, Morigiwa K (1988) Intraretinal axonsof ganglion cells in the Japanese monkey (Macaca fuscata): conduction velocity and diameter distribution. Neurosci Res 6:53–71
Ogden TE, Miller RF (1966) Studies of the optic nerve of the rhesus monkey: nerve fiber spectrum and physiological properties. Vision Res 6:485–506
Ogden TE (1984) Nerve fiber layer of the primate retina: morphometric analysis. Invest Ophthalmol Vis Sci 25:19–29
Ziccardi L, Sadun F, De Negri AM, Barboni P, Savini G, Borrelli E, La Morgia C, Carelli V, Parisi V (2013) Retinal function and neural conduction along the visual pathways in affected and unaffected carriers with Leber’s hereditary optic neuropathy. Invest Ophthalmol Vis Sci 54:6893–6901
Kurita-Tashima S, Tobimatsu S, Nakayama-Hiromatsu M, Kato M (1991) Effect of the check size on the pattern reversal visual evoked potential. Electroencephalogr Clin Neurophysiol 80:161–166
Parisi V, Scarale ME, Balducci N, Fresina M, Campos EC (2010) Electrophysiological detection of delayed postretinal neural conduction in human amblyopia. Invest Ophthalmol Vis Sci 51:5041–5048
Barboni P, Carbonelli M, Savini G, Ramos Cdo V, Carta A, Berezovsky A, Salomao SR, Carelli V, Sadun AA (2010) Natural history of Leber’s hereditary optic neuropathy: longitudinal analysis of the retinal nerve fiber layer by optical coherence tomography. Ophthalmology 117:623–627
Mashima Y, Imamura Y, Oguchi Y (1997) Dissociation of damage to spatial and luminance channels in early Leber’s hereditary optic neuropathy manifested by the visual evoked potential. Eye (London) 11:707–712
Sharkawi E, Oleszczuk JD, Holder GE, Raina J (2012) Clinical and electrophysiological recovery in Leber hereditary optic neuropathy with G3460A mutation. Doc Ophthalmol 125:71–74
Sadun AA, Win PH, Ross-Cisneros FN, Walker S, Carelli V (2000) Leber’s hereditary optic neuropathy differentially affects smaller axons in the optic nerve. Trans Am Ophthalmol Soc 98:223–232
Pan BX, Ross-Cisneros FN, Carelli V, Rue KS, Salomao SR, Moraes-Filho MN, Moraes MN, Berezovsky A, Belfort R Jr, Sadun AA (2012) Mathematically modeling the involvement of axons in Leber’s hereditary optic neuropathy. Invest Ophthalmol Vis Sci 53:7608–7617
Sadun AA, La Morgia C, Carelli V (2013) Mitochondrial optic neuropathies: our travels from bench to bedside and back again. Clin Experiment Ophthalmol 41:702–712
Sadun AA (1998) Acquired mitochondrial impairment as a cause of optic nerve disease. Trans Am Ophthalmol Soc 46:881–923
Procaccio V, Bris C, Chao de la Barca JM, Oca F, Chevrollier A, Amati-Bonneau P, Bonneau D, Reynier P (2014) Perspective of drug-based neuroprotection targeting mitochondria. Rev Neurol (Paris) 170:390–400
Lachenmayr BJ, Vivell PMO (1993) Principles of perimetry. In: Lachenmayr BJ, Vivell PMO (eds) Perimetry and its clinical correlation. Thieme Medical Publishers Inc., New York, pp 12–13
Moschos MM, Georgopoulos G, Chatziralli IP, Koutsandrea C (2012) Multifocal VEP and OCT findings in patients with primary open angle glaucoma: a cross-sectional study. BMC Ophthalmol 12:34
Acknowledgments
Research for this paper was financially supported partially by the Italian Ministry of Health (grant number: 2006 RF-FGB-2006-368547) and partially by Fondazione Roma. The authors acknowledge Dr. Valter Valli Fiore for technical help in electrophysiological evaluations.
Conflict of interest
Each author states that he/she has no proprietary interest in the development or marketing of the instruments used in the present study, and no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ziccardi, L., Parisi, V., Giannini, D. et al. Multifocal VEP provide electrophysiological evidence of predominant dysfunction of the optic nerve fibers derived from the central retina in Leber’s hereditary optic neuropathy. Graefes Arch Clin Exp Ophthalmol 253, 1591–1600 (2015). https://doi.org/10.1007/s00417-015-2979-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-015-2979-1