Abstract
Background
To evaluate risk factors for subject withdrawals from multicenter clinical trials evaluating glaucoma medications.
Methods
An analysis of prospective, randomized, multicenter, parallel, active-controlled clinical trials with 70 subjects/treatment arm published from 1996–2008.
Results
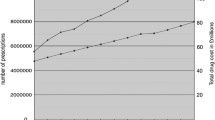
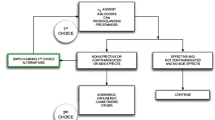
We analyzed 36 glaucoma studies including 17,511 subjects at 1,294 clinical sites. There were 2,060 (12%) subject withdrawals with 669 (32%) for administrative errors, 945 (46%) for adverse events (AEs), 197 (10%) for inadequate intraocular pressure (IOP) control and 249 (12%) for unknown reasons. By multilinear regression analysis, no positive risk factors for early subject withdrawals were observed following a Bonferroni correction (p ≥ 0.01). A positive correlation was observed for medication errors and protocol violations to withdrawals due to ocular AEs and total administrative errors (p < 0.0001). Protocol violations alone were correlated to subject withdrawals for any AE (total/month) and systemic AEs (p < 0.0001). Females and Caucasians were correlated to medication errors (p < 0 .0001). Among medical therapies, alpha-agonists, beta-blockers, the carbonic anhydrase inhibitor/beta-blocker fixed combination and prostaglandins were correlated with systemic AEs (p ≤ 0.005) while the alpha-agonists were correlated with withdrawals for poor IOP control (p = 0.00056).
Conclusions
Subject withdrawals from clinical trials for total administrative errors or AEs potentially might be reduced by choosing sites with lower historical rates of protocol violations or medication dispensing errors. Drug class choice also may influence subject withdrawals for AEs and poor IOP control.
Similar content being viewed by others
References
Silverman SL (2009) From randomized controlled trials to observational studies. Am J Med 122:114–120
Lai TY, Wong VW, Lam RF, Cheng AC, Lam DS, Leung GM (2007) Quality of reporting of key methodological items of randomized controlled trials in clinical ophthalmic journals. Ophthalmic Epidemiol 14:390–398
Lumley J, Bastian H (1996) Competing or complementary? Ethical considerations and the quality of randomized trials. Int J Technol Assess Health Care 12:247–263
Siegel S (1956) Nonparametric Statistics for the Behavioral Sciences. McGraw-Hill, Inc, New York
Miller RG 91998) Beyond ANOVA: Basics of Applied Statistics. Chapman & Hall/CRC, Boca Raton, pp 1–36
Book SA (1978) Essentials of Statistics. McGraw-Hill, Inc, New York
Stewart WC, Castelli WP (1996) Systemic side effects of topical β-adrenergic blockers. Clin Cardiol 19:691–697
Stewart WC, Garrison PM (1998) ß-blocker-induced complications and the glaucoma subject: newer treatments to help reduce systemic side effects. Arch Intern Med 158:221–226
Stewart WC, Stewart JA, Jackson AL (2002) Cardiovascular effects of timolol maleate, brimonidine or brimonidine/timolol maleate in concomitant therapy. Acta Ophthalmol Scand 80:277–281
Alm A, Grierson I, Shields MB (2008) Side effects associated with prostaglandin analog therapy. Surv Ophthalmol 53(Suppl1):S93–105
Stewart WC, Day DG, Stewart JA, Schuhr J, Latham KE (2001) The efficacy and safety of latanoprost 0.005% once daily versus brimonidine 0.2% twice daily in open-angle glaucoma or ocular hypertension. Am J Ophthalmol 131:631–635
Barsky AJ, Peekna HM, Borus JF (2001) Somatic symptom reporting in women and men. J Gen Intern Med 16:266–275
Kroenke K, Spitzer RL (1998) Gender differences in the reporting of physical and somatoform symptoms. Psychosom Med 60:150–155
Author information
Authors and Affiliations
Corresponding author
Additional information
PRN Pharmaceutical Research Network, LLC, received no financial support from any private or government funding source. The authors do not have any proprietary interest to disclose.
Electronic supplementary materials
Below is the link to the electronic supplementary material.
ESM Table 1
(DOC 61 kb)
Rights and permissions
About this article
Cite this article
Stewart, W.C., Demos, C.M., Turner, M.K. et al. Risk factors for subject withdrawals in clinical trials evaluating glaucoma medications. Graefes Arch Clin Exp Ophthalmol 248, 1007–1012 (2010). https://doi.org/10.1007/s00417-010-1339-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-010-1339-4