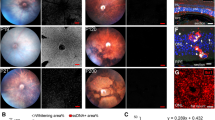
Abstract
Background
Bone spicule pigments (BSP) are a hallmark of retinitis pigmentosa (RP). In this study, we examined the process of BSP formation in the rhodopsin knockout (rho -/-) mouse, a murine model for human RP.
Methods
In rho -/- mice from 2 to 16 months of age, representing the range from early to late stages of degeneration, retinal sections and whole mounts were examined morphologically by light and electron microscopy. The results were compared to scanning laser ophthalmoscopy of BSP degeneration in human RP.
Results
After the loss of all photoreceptor cells in rho-/- mice, the outer retina successively degenerated, leading to approximation and finally a direct contact of inner retinal vessels and the retinal pigment epithelium (RPE). We could show that it was the event of proximity of retinal vessel and RPE that triggered migration of RPE cells along the contacting vessels towards the inner retina. Ultrastructurally, these mislocalized RPE cells partially sealed the vessels by tight junction linkage and deposited extracellular matrix perivascularly. Also, the vascular endothelium developed fenestrations similar to the RPE-choroid interface. In whole mounts, the pigmented cell clusters outlining retinal capillaries correlated well with BSPs in human RP. The structure of the inner retina remained well preserved, even in late stages.
Conclusions
The Rho -/- mouse is the first animal model that depicts all major pathological changes, even in the late stages of RP. Using the rho -/- mouse model we were able to analyze the complete dynamic process of BSP formation. Therefore we conclude that: (1) In rho -/- retinas, BSPs only form in areas devoid of photoreceptors; (2) Direct contact between inner retinal vessels and RPE appears to be a major trigger for migration of RPE cells; (3) The distribution of the RPE cells in BSPs reflects the vascular network at the time of formation. The similarity of the disease process between mouse and human and the possibility to study all consecutive steps of the course of the disease makes the rho -/- mouse valuable for further insights in the dynamics of BSP formation in human RP.





Similar content being viewed by others
References
Hartong DT, Berson EL, Dryja TP (2006) Retinitis pigmentosa. Lancet 368:1795–1809
Pagon RA (1988) Retinitis pigmentosa. Surv Ophthalmol 33:137–177
Milam AH, Li ZY, Fariss RN (1998) Histopathology of the human retina in retinitis pigmentosa. Prog Retin Eye Res 17:175–205
Santos A, Humayun MS, de Juan E, Greenburg RJ, Marsh MJ, Klock IB, Milam AH (1997) Preservation of the inner retina in retinitis pigmentosa. A morphometric analysis. Arch Ophthalmol 115:511–515
Lahav M, Craft J, Albert DM, Ishii Y (1982) Advanced pigmentary retinal degeneration. An ultrastructural study. Retina 2:65–75
Milam AH, Kalina RE, Pagon RA (1983) Clinical-ultrastructural study of a retinal dystrophy. Invest Ophthalmol Vis Sci 24:458–469
Szamier RB, Berson EL (1977) Retinal ultrastructure in advanced retinitis pigmentosa. Invest Ophthalmol Vis Sci 16:947–962
Milam AH, De Castro EB, Smith JE, Tang WX, John SJ, Gorin MB, Stone EM, Aguirre GD, Jacobson SG (2001) Concentric retinitis pigmentosa: clinicopathologic correlations. Exp Eye Res 73:493–508
Li ZY, Jacobson SG, Milam AH (1994) Autosomal dominant retinitis pigmentosa caused by the threonine-17-methionine rhodopsin mutation: retinal histopathology and immunocytochemistry. Exp Eye Res 58:397–408
Li ZY, Possin DE, Milam AH (1995) Histopathology of bone spicule pigmentation in retinitis pigmentosa. Ophthalmology 102:805–816
To K, Adamian M, Dryja TP, Berson EL (2002) Histopathologic study of variation in severity of retinitis pigmentosa due to the dominant rhodopsin mutation Pro23His. Am J Ophthalmol 134:290–293
Flannery JG, Farber DB, Bird AC, Bok D (1989) Degenerative changes in a retina affected with autosomal dominant retinitis pigmentosa. Invest Ophthalmol Vis Sci 30:191–211
Humphries MM, Rancourt D, Farrar GJ, Kenna P, Hazel M, Bush RA, Sieving PA, Sheils DM, McNally N, Creighton P, Erven A, Boros A, Gulya K, Capecchi MR, Humphries P (1997) Retinopathy induced in mice by targeted disruption of the rhodopsin gene. Nat Genet 15:216–219
Toda K, Bush RA, Humphries P, Sieving PA (1999) The electroretinogram of the rhodopsin knockout mouse. Vis Neurosci 16:391–398
Jaissle GB, May CA, Reinhard J, Kohler K, Fauser S, Lütjen-Drecoll E, Zrenner E, Seeliger MW (2001) Evaluation of the rhodopsin knockout mouse as a model of pure cone function. Invest Ophthalmol Vis Sci 42:506–513
Humphries MM, Kiang S, McNally N, Donovan MA, Sieving PA, Bush RA, Machida S, Cotter T, Hobson A, Farrar J, Humphries P, Kenna P (2001) Comparative structural and functional analysis of photoreceptor neurons of Rho-/- mice reveal increased survival on C57BL/6J in comparison to 129 Sv genetic background. Vis Neurosci 18:437–443
Hobson AH, Donovan M, Humphries MM, Tuohy G, McNally N, Carmody R, Cotter T, Farrar GJ, Kenna PF, Humphries P (2000) Apoptotic photoreceptor death in the rhodopsin knockout mouse in the presence and absence of c-fos. Exp Eye Res 71:247–254
Campbell M, Humphries M, Kennan A, Kenna P, Humphries P, Brankin B (2006) Aberrant retinal tight junction and adherens junction protein expression in an animal model of autosomal dominant Retinitis pigmentosa: the Rho(-/-) mouse. Exp Eye Res 83:484–492
Nishikawa S, LaVail MM (1998) Neovascularization of the RPE: temporal differences in mice with rod photoreceptor gene defects. Exp Eye Res 67:509–515
Wolfrum U (1991) Centrin- and alpha-actinin-like immunoreactivity in the ciliary rootlets of insect sensilla. Cell Tissue Res 266:31–38
Reiners J, Reidel B, El-Amraoui A, Boëda B, Huber I, Petit C, Wolfrum U (2003) Differential distribution of harmonin isoforms and their possible role in Usher 1 protein complexes in mammalian photoreceptor cells. Invest Ophthalmol Vis Sci 44:5006–5015
Penn JS, Li S, Naash MI (2000) Ambient hypoxia reverses retinal vascular attenuation in a transgenic mouse model of autosomal dominant retinitis pigmentosa. Invest Ophthalmol Vis Sci 41:4007–4013
Blaauwgeers HG, Holtkamp GM, Rutten H, Witmer AN, Koolwijk P, Partanen TA, Alitalo K, Kroon ME, Kijlstra A, van Hinsbergh VW, Schlingemann RO (1999) Polarized vascular endothelial growth factor secretion by human retinal pigment epithelium and localization of vascular endothelial growth factor receptors on the inner choriocapillaris. Evidence for a trophic paracrine relation. Am J Pathol 155:421–428
Roque RS, Caldwell RB (1991) Pigment epithelial cell changes precede vascular transformations in the dystrophic rat retina. Exp Eye Res 53:787–798
Ida H, Tobe T, Nambu H, Matsumura M, Uyama M, Campochiaro PA (2003) RPE cells modulate subretinal neovascularization, but do not cause regression in mice with sustained expression of VEGF. Invest Ophthalmol Vis Sci 44:5430–5437
Acknowledgements
The authors thank Dr. Dominik Fischer for the SLO investigation.
Financial interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by DFG grants Se837/1–1, 1–2, 4–1,6–1, Wo 548/6–2, and BMBF grant 0314106.
The authors have full control of all primary data and they agree to allow Graefe′s Archive for Clinical and Experimental Ophthalmology to review their data upon request.
Gesine B. Jaissle and Christian Albrecht May contributed equally to this work
An erratum to this article can be found at http://dx.doi.org/10.1007/s00417-010-1357-2
Rights and permissions
About this article
Cite this article
Jaissle, G.B., May, C.A., van de Pavert, S.A. et al. Bone spicule pigment formation in retinitis pigmentosa: insights from a mouse model. Graefes Arch Clin Exp Ophthalmol 248, 1063–1070 (2010). https://doi.org/10.1007/s00417-009-1253-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-009-1253-9