Abstract
Background
Altered thalamic volumes and resting state (RS) functional connectivity (FC) might be associated with physical activity (PA) and cardiorespiratory fitness (CRF) in people with progressive multiple sclerosis (PMS).
Objectives
To assess thalamic structural and functional alterations and investigate their correlations with PA/CRF levels in people with PMS.
Methods
Seven-day accelerometry and cardiopulmonary exercise testing were used to assess PA/CRF levels in 91 persons with PMS. They underwent 3.0 T structural and RS fMRI acquisition with 37 age/sex-matched healthy controls (HC). Between-group comparisons of MRI measures and their correlations with PA/CRF variables were assessed.
Results
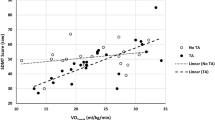
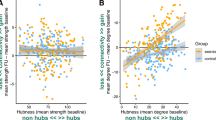
PMS people had lower volumes compared to HC (all p < 0.001). At corrected threshold, PMS showed decreased intra- and inter-thalamic RS FC, and increased RS FC between the thalamus and the hippocampus, bilaterally. At uncorrected threshold, decreased thalamic RS FC with caudate nucleus, cerebellum and anterior cingulate cortex (ACC), as well as increased thalamic RS FC with occipital regions, were also detected. Lower CRF, measured as peak oxygen consumption (VO2peak), correlated with lower white matter volume (r = 0.31, p = 0.03). Moreover, lower levels of light PA correlated with increased thalamic RS FC with the right hippocampus (r = − 0.3, p = 0.05).
Discussion
People with PMS showed widespread brain atrophy, as well as pronounced intra-thalamic and thalamo-hippocampal RS FC abnormalities. White matter atrophy correlated with CRF, while increased thalamo-hippocampal RS FC was associated to worse PA levels. Thalamic RS FC might be used to monitor physical impairment and efficacy of rehabilitative and disease-modifying treatments in future studies.

Similar content being viewed by others
Data availability
The anonymised dataset used and analysed during the current study is available from the corresponding author upon reasonable request.
References
Reich DS, Lucchinetti CF, Calabresi PA (2018) Multiple Sclerosis. N Engl J Med 378:169–180
Lublin FD, Coetzee T, Cohen JA, Marrie RA, Thompson AJ, Trials IACC (2020) The 2013 clinical course descriptors for multiple sclerosis A clarification. Neurology 94:1088–1092
Marck CH, Hadgkiss EJ, Weiland TJ, van der Meer DM, Pereira NG, Jelinek GA (2014) Physical activity and associated levels of disability and quality of life in people with multiple sclerosis: a large international survey. BMC Neurol 14:143
Feinstein A, Freeman J, Lo AC (2015) Progressive multiple sclerosis 2 Treatment of progressive multiple sclerosis: what works, what does not, and what is needed. Lancet Neurol 14:194–207
Langeskov-Christensen M, Heine M, Kwakkel G, Dalgas U (2015) Aerobic capacity in persons with multiple sclerosis: a systematic review and meta-analysis. Sports Med 45:905–923
Kinnett-Hopkins D, Adamson B, Rougeau K, Motl RW (2017) People with MS are less physically active than healthy controls but as active as those with other chronic diseases: An updated meta-analysis. Mult Scler Relat Dis 13:38–43
Stuart CM, Varatharaj A, Domjan J, Philip S, Galea I, Grp SS (2020) Physical activity monitoring to assess disability progression in multiple sclerosis. Mult Scler J-Exp Tra 6:205521732097518
Block VJ, Bove R, Zhao C et al (2019) Association of continuous assessment of step count by remote monitoring with disability progression among adults with multiple sclerosis. Jama Netw Open 2:e190570
Negaresh R, Motl RW, Zimmer P, Mokhtarzade M, Baker JS (2019) Effects of exercise training on multiple sclerosis biomarkers of central nervous system and disease status: a systematic review of intervention studies. Eur J Neurol 26:711–721
Prakash RS, Snook EM, Motl RW, Kramer AF (2010) Aerobic fitness is associated with gray matter volume and white matter integrity in multiple sclerosis. Brain Res 1341:41–51
Motl RW, Pilutti LA, Hubbard EA, Wetter NC, Sosnoff JJ, Sutton BP (2015) Cardiorespiratory fitness and its association with thalamic, hippocampal, and basal ganglia volumes in multiple sclerosis. Neuroimage-Clin 7:661–666
Klaren RE, Hubbard EA, Motl RW, Pilutti LA, Wetter NC, Sutton BP (2015) Objectively measured physical activity is associated with brain volumetric measurements in multiple sclerosis. Behav Neurol
Negaresh R, Gharakhanlou R, Sahraian MA, Abolhasani M, Motl RW, Zimmer P (2021) Physical activity may contribute to brain health in multiple sclerosis: An MR volumetric and spectroscopy study. J Neuroimaging 31:714–723
Sandroff BM, Motl RW, Amato MP et al (2021) Cardiorespiratory fitness and free-living physical activity are not associated with cognition in persons with progressive multiple sclerosis: Baseline analyses from the CogEx study. Mult Scler J 28:1091–1110
Zackowski KM, Freeman J, Brichetto G et al (2021) Prioritizing progressive MS rehabilitation research: A call from the International Progressive MS Alliance. Mult Scler J 27:989–1001
Rocca MA, Valsasina P, Leavitt VM et al (2018) Functional network connectivity abnormalities in multiple sclerosis: Correlations with disability and cognitive impairment. Mult Scler 24:459–471
Minagar A, Barnett MH, Benedict RHB et al (2013) The thalamus and multiple sclerosis Modern views on pathologic, imaging, and clinical aspects. Neurology 80:210–219
d’Ambrosio A, Hidalgo de la Cruz M, Valsasina P et al (2017) Structural connectivity-defined thalamic subregions have different functional connectivity abnormalities in multiple sclerosis patients: Implications for clinical correlations. Hum Brain Mapp 38:6005–6018
Hidalgo de la Cruz M, Valsasina P, Mesaros S et al (2021) Clinical predictivity of thalamic sub-regional connectivity in clinically isolated syndrome: a 7-year study. Mol Psychiatry 26:2163–2174
Liu Y, Liang P, Duan Y et al (2015) Altered thalamic functional connectivity in multiple sclerosis. Eur J Radiol 84:703–708
Schoonheim MM, Hulst HE, Brandt RB et al (2015) Thalamus structure and function determine severity of cognitive impairment in multiple sclerosis. Neurology 84:776–783
Zhou F, Gong H, Chen Q et al (2016) Intrinsic functional plasticity of the thalamocortical system in minimally disabled patients with relapsing-remitting multiple sclerosis. Front Hum Neurosci 10:2
De Giglio L, Tona F, De Luca F et al (2016) Multiple sclerosis: changes in thalamic resting-state functional connectivity induced by a home-based cognitive rehabilitation program. Radiology 280:202–211
Tona F, Petsas N, Sbardella E et al (2014) Multiple sclerosis: altered thalamic resting-state functional connectivity and its effect on cognitive function. Radiology 271:814–821
Schoonheim MM, Pinter D, Prouskas SE et al (2022) Disability in multiple sclerosis is related to thalamic connectivity and cortical network atrophy. Mult Scler J 28:61–70
Hidalgo de la Cruz M, d’Ambrosio A, Valsasina P et al (2018) Abnormal functional connectivity of thalamic sub-regions contributes to fatigue in multiple sclerosis. Mult Scler 24:1183–1195
Feinstein A, Amato MP, Brichetto G et al (2020) Study protocol: improving cognition in people with progressive multiple sclerosis: a multi-arm, randomized, blinded, sham-controlled trial of cognitive rehabilitation and aerobic exercise (COGEx). BMC Neurol 20:204
Lublin FD, Reingold SC, Cohen JA et al (2014) Defining the clinical course of multiple sclerosis The 2013 revisions. Neurology 83:278–286
Motl RW, Bollaert RE, Sandroff BM (2018) Validation of the godin leisure-time exercise questionnaire classification coding system using accelerometry in multiple sclerosis. Rehabil Psychol 63:77–82
Strober L, DeLuca J, Benedict RHB et al (2019) Symbol digit modalities test: a valid clinical trial endpoint for measuring cognition in multiple sclerosis. Mult Scler J 25:1781–1790
Kurtzke JF (1983) Rating neurologic impairment in multiple-sclerosis—an Expanded Disability Status Scale (Edss). Neurology 33:1444–1452
Goldman MD, Marrie RA, Cohen JA (2008) Evaluation of the six-minute walk in multiple sclerosis subjects and healthy controls. Mult Scler J 14:383–390
Pilutti LA, Sandroff BM, Klaren RE et al (2015) Physical fitness assessment across the disability spectrum in persons with multiple sclerosis: a comparison of testing modalities. J Neurol Phys Therapy 39:241–249
Valverde S, Cabezas M, Roura E et al (2017) Improving automated multiple sclerosis lesion segmentation with a cascaded 3D convolutional neural network approach. Neuroimage 155:159–168
Smith SM, Jenkinson M, Woolrich MW et al (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23:S208–S219
Whitfield-Gabrieli S, Nieto-Castanon A (2012) Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2:125–141
Behzadi Y, Restom K, Liau J, Liu TT (2007) A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37:90–101
Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA (2017) FMRI clustering in AFNI: false-positive rates redux. Brain Connect 7:152–171
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B (Methodol) 57:289–300
Tewarie P, Steenwijk MD, Brookes MJ et al (2018) Explaining the heterogeneity of functional connectivity findings in multiple sclerosis: an empirically informed modeling study. Hum Brain Mapp 39:2541–2548
Goldstone A, Mayhew SD, Hale JR, Wilson RS, Bagshaw AP (2018) Thalamic functional connectivity and its association with behavioral performance in older age. Brain and Behavior 8:e00943
Sexton CE, Betts JF, Demnitz N, Dawes H, Ebmeier KP, Johansen-Berg H (2016) A systematic review of MRI studies examining the relationship between physical fitness and activity and the white matter of the ageing brain. Neuroimage 131:81–90
Proschinger S, Kuhwand P, Rademacher A et al (2022) Fitness, physical activity, and exercise in multiple sclerosis: a systematic review on current evidence for interactions with disease activity and progression. J Neurol 269:2922–2940
Diechmann MD, Campbell E, Coulter E, Paul L, Dalgas U, Hvid LG (2021) Effects of exercise training on neurotrophic factors and subsequent neuroprotection in persons with multiple sclerosis-a systematic review and meta-analysis. Brain Sci 11:1499
Dalgas U, Langeskov-Christensen M, Stenager E, Riemenschneider M, Hvid LG (2019) Exercise as medicine in multiple sclerosis-time for a paradigm shift: preventive, symptomatic, and disease-modifying aspects and perspectives. Curr Neurol Neurosci 19:88
Won J, Callow DD, Pena GS et al (2021) Evidence for exercise-related plasticity in functional and structural neural network connectivity. Neurosci Biobehav R 131:923–940
Hulst HE, Schoonheim MM, Van Geest Q, Uitdehaag BM, Barkhof F, Geurts JJ (2015) Memory impairment in multiple sclerosis: Relevance of hippocampal activation and hippocampal connectivity. Mult Scler 21:1705–1712
van Geest Q, Hulst HE, Meijer KA, Hoyng L, Geurts JJG, Douw L (2018) The importance of hippocampal dynamic connectivity in explaining memory function in multiple sclerosis. Brain Behav 8:e00954
Rocca MA, Pravata E, Valsasina P et al (2015) Hippocampal-DMN disconnectivity in MS is related to WM lesions and depression. Hum Brain Mapp 36:5051–5063
Macdonald E, Buchan D, Cerexhe L, Renfrew L, Sculthorpe N (2023) Accelerometer measured physical activity and sedentary time in individuals with multiple sclerosis versus age matched controls: a systematic review and meta-analysis. Mult Scler Relat Dis 69:104462
Sandroff BM, Wylie GR, Sutton BP, Johnson CL, DeLuca J, Motl RW (2018) Treadmill walking exercise training and brain function in multiple sclerosis: preliminary evidence setting the stage for a network-based approach to rehabilitation. Mult Scler J Exp Transl Clin 4:2055217318760641
Huiskamp M, Moumdjian L, van Asch P et al (2020) A pilot study of the effects of running training on visuospatial memory in MS: A stronger functional embedding of the hippocampus in the default-mode network? Mult Scler 26:1594–1598
Sandroff BM, Motl RW, Kam JP, Pula JH (2014) Accelerometer measured physical activity and the integrity of the anterior visual pathway in multiple sclerosis. Mult Scler Relat Dis 3:117–122
Ahmed J, Stephens S, Ly M, Longoni G, Yeh EA (2021) Structural visual metrics associate with moderate to vigorous physical activity in youth with pediatric-onset neuroinflammatory disorders. Mult Scler J 27:496–497
Bento-Torres J, Bento-Torres NVO, Stillman CM et al (2019) Associations between cardiorespiratory fitness, physical activity, intraindividual variability in behavior, and cingulate cortex in younger adults. J Sport Health Sci 8:315–324
Bland JM, Altman DG (2011) Correlation in restricted ranges of data. Bmj-Brit Med J 342:d556
Vul E, Harris C, Winkielman P, Pashler H (2009) Puzzlingly High Correlations in fMRI Studies of Emotion, Personality, and Social Cognition. Perspect Psychol Sci 4:274–290
Acknowledgments
The authors would like to thank all the centers and their staff members that were involved in the study.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by a grant from the Multiple Sclerosis Society of Canada (grant no. #EGID3185) and the National MS Society.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflicts of interest
Francesco Romanò has nothing to disclose; Robert W. Motl has nothing to disclose; Paola Valsasina received speaker honoraria from Biogen Idec; Maria Pia Amato received compensation for consulting services and/or speaking activities from Bayer, Biogen Idec, Merck-Serono, Novartis, Roche, Sanofi Genzyme, and Teva Pharmaceutical Industries; and receives research support from Biogen Idec, Merck-Serono, Roche, Pharmaceutical Industries and Fondazione Italiana Sclerosi Multipla; Giampaolo Brichetto has been awarded and receives research support from Roche, Fondazione Italiana Sclerosi Multipla, ARSEP, H2020 EU Call; Nicolò Bruschi has nothing to disclose; Jeremy Chataway has received support from the Efficacy and Evaluation (EME) Programme, a Medical Research Council (MRC) and National Institute for Health Research (NIHR) partnership and the Health Technology Assessment (HTA) Programme (NIHR), the UK MS Society, the US National MS Society and the Rosetrees Trust, he is supported in part by the National Institute for Health Research, University College London Hospitals, Biomedical Research Centre, London, UK, he has been a local principal investigator for commercial trials funded by Actelion, Biogen, Novartis and Roche, has received an investigator grant from Novartis, and has taken part in advisory boards/consultancy for Azadyne, Biogen, Celgene, MedDay, Merck and Roche; Nancy D. Chiaravalloti is on an Advisory Board for Akili Interactive and is a member of the Editorial Boards of Multiple Sclerosis Journal and Frontiers in NeuroTrauma; Gary Cutter is a member of Data and Safety Monitoring Boards for Astra-Zeneca, Avexis Pharmaceuticals, Biolinerx, Brainstorm Cell Therapeutics, Bristol Meyers Squibb/Celgene, CSL Behring, Galmed Pharmaceuticals, Horizon Pharmaceuticals, Hisun Pharmaceuticals, Mapi Pharmaceuticals LTD, Merck, Merck/Pfizer, Opko Biologics, OncoImmune, Neurim, Novartis, Ophazyme, Sanofi Aventis, Reata Pharmaceuticals, Teva pharmaceuticals, VielaBio Inc, Vivus, NHLBI (Protocol Review Committee), NICHD (OPRU oversight committee), he is on Consulting or Advisory Boards for Biodelivery Sciences International, Biogen, Click Therapeutics, Genzyme, Genentech, GW Pharmaceuticals, Klein-Buendel Incorporated, Medimmune, Medday, Neurogenesis LTD, Novartis, Osmotica Pharmaceuticals, Perception Neurosciences, Recursion/Cerexis Pharmaceuticals, Roche, TG Therapeutics, he is employed by the University of Alabama at Birmingham and President of Pythagoras, Inc, a private consulting company located in Birmingham AL; Ulrik Dalgas has received research support, travel grants, and/or teaching honorary from Biogen Idec, Merck-Serono, Novartis, Bayer Schering, and Sanofi Aventis as well as honoraria from serving on scientific advisory boards of Biogen Idec and Genzyme; John DeLuca is an Associate Editor of the Archives of Physical Medicine, received compensation for consulting services and/or speaking activities from Biogen Idec, Celgene/Bristol Myers Squibb, MedRhythms, Janssen, and Novartis, and receives research support from Biogen Idec, Genzyme, Bristol Myers Squibb, National Multiple Sclerosis Society, Consortium of Multiple Sclerosis Centers, and National Institutes of Health; Rachel Farrell has received honoraria and served on advisory panels for Merck, TEVA, Novartis, Genzyme, GW pharma (Jazz pharmaceuticals), Allergan, Merz, Ipsen and Biogen. She is supported in part by the National Institute for Health Research, University College London Hospitals, Biomedical Research Centre, London, UK; Peter Feys is editorial board member of NNR, MSJ and Frontiers in Rehabilitation Sciences (section ‘Strengthening Health Systems’), provides consultancy to NeuroCompass and was board of advisory board meetings for BIOGEN; Jennifer Freeman has been awarded research grants from the NIHR, UK; Matilde Inglese is Co-Editor for Controversies for Multiple Sclerosis Journal, received compensation for consulting services and/or speaking activities from Biogen Idec, Merck-Serono, Novartis, Roche, Sanofi Genzyme, and received research support from NIH, NMSS, the MS Society of Canada, the Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla, H2020 EU Call; Cecilia Meza has nothing to disclose; Amber Salter receives research funding from Multiple Sclerosis Society of Canada, National Multiple Sclerosis Society, CMSC and the US Department of Defense and is a member of editorial board for Neurology; Brian Sandroff has nothing to disclose; Anthony Feinstein is on Advisory Boards for Akili Interactive and Roche, and reports grants from the MS Society of Canada, book royalties from Johns Hopkins University Press, Cambridge University Press, Amadeus Press and Glitterati Editions, and speaker’s honoraria from Novartis, Biogen, Roche and Sanofi Genzyme; Maria Assunta Rocca received consulting fees from Biogen, Bristol Myers Squibb, Eli Lilly, Janssen, Roche; and speaker honoraria from Bayer, Biogen, Bristol Myers Squibb, Bromatech, Celgene, Genzyme, Merck Healthcare Germany, Merck Serono SpA, Novartis, Roche, and Teva. She receives research support from the MS Society of Canada and Fondazione Italiana Sclerosi Multipla. She is Associate Editor for Multiple Sclerosis and Related Disorders; Massimo Filippi is Editor-in-Chief of the Journal of Neurology, Associate Editor of Human Brain Mapping, Associate Editor of Radiology, and Associate Editor of Neurological Sciences; received compensation for consulting services from Alexion, Almirall, Biogen, Merck, Novartis, Roche, Sanofi; speaking activities from Bayer, Biogen, Celgene, Chiesi Italia SpA, Eli Lilly, Genzyme, Janssen, Merck-Serono, Neopharmed Gentili, Novartis, Novo Nordisk, Roche, Sanofi, Takeda, and TEVA; participation in Advisory Boards for Alexion, Biogen, Bristol-Myers Squibb, Merck, Novartis, Roche, Sanofi, Sanofi-Aventis, Sanofi-Genzyme, Takeda; scientific direction of educational events for Biogen, Merck, Roche, Celgene, Bristol-Myers Squibb, Lilly, Novartis, Sanofi-Genzyme; he receives research support from Biogen Idec, Merck-Serono, Novartis, Roche, Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla, and ARiSLA (Fondazione Italiana di Ricerca per la SLA).
Ethical approval
The study was approved by local research ethics committees or institutional review boards at enrolling sites. All participants provided written informed consent prior to study participation according to the Declaration of Helsinki.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Romanò, F., Motl, R.W., Valsasina, P. et al. Abnormal thalamic functional connectivity correlates with cardiorespiratory fitness and physical activity in progressive multiple sclerosis. J Neurol 270, 3213–3224 (2023). https://doi.org/10.1007/s00415-023-11664-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-023-11664-8