Abstract
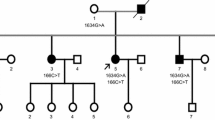
Amyotrophic lateral sclerosis type 4 (ALS4) is a familial form of ALS caused by mutations in the SETX gene. To date, there are seven unrelated ALS4 families with four missense mutations (L389S, T31I, R2136H, and M386T) in SETX. ALS4 is characterized by early onset, distal muscle weakness and atrophy, pyramidal signs, and the absence of sensory deficits. Motor conduction studies often present normality or reduced amplitudes of compound muscle action potential (CMAP). The conduction blocks (CBs) are rare and only observed in one male of an Italian ALS4 family. Our study showed that seven symptomatic patients presented the classical ALS4 phenotype with two asymptomatic females in a Chinese family spanning three generations. Sequencing analysis revealed a heterozygous c.1166T > C/p.L389S mutation in SETX that co-segregated with disease phenotype in the family. The same mutation has been identified previously in three ALS4 families from the United States and Italy, respectively. Specifically, three young males presented multiple CBs and abnormal temporal dispersions (TD) in the median, ulnar and tibial nerves over the three-year follow-up period. Moreover, for the first time, we found that senataxin was also expressed in the myelin sheath of peripheral nerves besides axons. The study indicates that CBs and abnormal TD are the characteristics in the ALS4 family, providing pivotal familial evidence of CBs and TD of motor nerves in ALS4. The unusual electrophysiological features may be associated with the expression of senataxin in peripheral nerves.



Similar content being viewed by others
References
van Es MA, Hardiman O, Chio A, Al-Chalabi A, Pasterkamp RJ, Veldink JH, van den Berg LH (2017) Amyotrophic lateral sclerosis. The Lancet 390(10107):2084–2098. https://doi.org/10.1016/s0140-6736(17)31287-4
Chance PF, Rabin BA, Ryan SG, Ding Y, Scavina M, Crain B, Griffin JW, Cornblath DR (1998) Linkage of the gene for an autosomal dominant form of juvenile amyotrophic lateral sclerosis to chromosome 9q34. Am J Hum Genet 62(3):633–640. https://doi.org/10.1086/301769
Chen YZ, Bennett CL, Huynh HM, Blair IP, Puls I, Irobi J, Dierick I, Abel A, Kennerson ML, Rabin BA, Nicholson GA, Auer-Grumbach M, Wagner K, De Jonghe P, Griffin JW, Fischbeck KH, Timmerman V, Cornblath DR, Chance PF (2004) DNA/RNA helicase gene mutations in a form of juvenile amyotrophic lateral sclerosis (ALS4). Am J Hum Genet 74(6):1128–1135. https://doi.org/10.1086/421054
Avemaria F, Lunetta C, Tarlarini C, Mosca L, Maestri E, Marocchi A, Melazzini M, Penco S, Corbo M (2011) Mutation in the senataxin gene found in a patient affected by familial ALS with juvenile onset and slow progression. Amyotroph Lateral Scler 12(3):228–230
Rudnik-Schöneborn S, Arning L, Epplen JT, Zerres K (2012) SETX gene mutation in a family diagnosed autosomal dominant proximal spinal muscular atrophy. Neuromuscul Disord 22(3):258–262. https://doi.org/10.1016/j.nmd.2011.09.006
Ma L, Shi Y, Chen Z, Li S, Zhang J (2018) A novel SETX gene mutation associated with Juvenile amyotrophic lateral sclerosis. Brain Behav 8(9):e01066. https://doi.org/10.1002/brb3.1066
Taniguchi T, Hokezu Y, Okada T, Ishibashi M, Hashiguchi A, Matsuura E, Takashima H (2017) A amyotrophic lateral sclerosis (ALS) 4 family misdiagnosed as hereditary spastic paraplegia-a case report-. Rinsho Shinkeigaku 57(11):685–690. https://doi.org/10.5692/clinicalneurol.cn-000996
Rabin BA, Griffin JW, Crain BJ, Scavina M, Chance PF, Cornblath DR (1999) Autosomal dominant juvenile amyotrophic lateral sclerosis. Brain 122(Pt 8):1539–1550. https://doi.org/10.1093/brain/1122.1538.1539
De Jonghe P, Auer-Grumbach M, Irobi J, Wagner K, Plecko B, Kennerson M, Zhu D, De Vriendt E, Van Gerwen V, Nicholson G, Hartung HP, Timmerman V (2002) Autosomal dominant juvenile amyotrophic lateral sclerosis and distal hereditary motor neuronopathy with pyramidal tract signs: synonyms for the same disorder? Brain 125(Pt 6):1320–1325. https://doi.org/10.1093/brain/awf127
Mallik A (2005) Nerve conduction studies: essentials and pitfalls in practice. J Neurol Neurosurg Psychiatry 76(suppl_2):ii23–ii31. https://doi.org/10.1136/jnnp.2005.069138
Olney RK (1999) Guidelines in electrodiagnostic medicine. Consensus criteria for the diagnosis of partial conduction block. Muscle Nerve Suppl 8:S225–S229
Weber F (1997) Conduction block and abnormal temporal dispersion–diagnostic criteria. Electromyogr Clin Neurophysiol 37(5):305–309
Sun Y, Chen H, Lu Y, Duo J, Lei L, OuYang Y, Hao Y, Da Y, Shen XM (2019) Limb girdle muscular dystrophy D3 HNRNPDL related in a Chinese family with distal muscle weakness caused by a mutation in the prion-like domain. J Neurol 266(2):498–506. https://doi.org/10.1007/s00415-018-9165-4
Chen YZ, Hashemi SH, Anderson SK, Huang Y, Moreira MC, Lynch DR, Glass IA, Chance PF, Bennett CL (2006) Senataxin, the yeast Sen1p orthologue: characterization of a unique protein in which recessive mutations cause ataxia and dominant mutations cause motor neuron disease. Neurobiol Dis 23(1):97–108. https://doi.org/10.1016/j.nbd.2006.02.007
Vantaggiato C, Bondioni S, Airoldi G, Bozzato A, Borsani G, Rugarli EI, Bresolin N, Clementi E, Bassi MT (2011) Senataxin modulates neurite growth through fibroblast growth factor 8 signalling. Brain 134(Pt 6):1808–1828. https://doi.org/10.1093/brain/awr084
Wen J, Li L, Tan D, Guo J (2017) Preparation of teased nerve fibers from rat sciatic nerve. Bio-Protocol 7(19):e2572. https://doi.org/10.21769/BioProtoc.2572
Taylor BV, Dyck PJ, Engelstad J, Gruener G, Grant I (2004) Multifocal motor neuropathy: pathologic alterations at the site of conduction block. J Neuropathol Exp Neurol 63(2):129–137. https://doi.org/10.1093/jnen/63.2.129
Hu B, McCollum M, Ravi V, Arpag S, Moiseev D, Castoro R, Mobley B, Burnette B, Siskind C, Day J, Yawn R, Feely S, Li Y, Yan Q, Shy M, Li J (2018) Myelin abnormality in Charcot-Marie-Tooth type 4 J recapitulates features of acquired demyelination. Ann Neurol 83(4):756–770. https://doi.org/10.1002/ana.25198
Groh M, Albulescu LO, Cristini A, Gromak N (2017) Senataxin: genome guardian at the interface of transcription and neurodegeneration. J Mol Biol 429(21):3181–3195. https://doi.org/10.1016/j.jmb.2016.3110.3021
Grunseich C, Wang IX, Watts JA, Burdick JT, Guber RD, Zhu Z, Bruzel A, Lanman T, Chen K, Schindler AB, Edwards N, Ray-Chaudhury A, Yao J, Lehky T, Piszczek G, Crain B, Fischbeck KH, Cheung VG (2018) senataxin mutation reveals how r-loops promote transcription by blocking DNA methylation at gene promoters. Mol Cell 69(3):426–437. https://doi.org/10.1016/j.molcel.2017.1012.1030e427
Acknowledgments
The authors thank technician Zhongxi Lin, Dr. Deqiang Han, and Dr. Yu Zhao for technical assistance, and Dr. Dingguo Shen for neurophysiological help. The research was supported by National Key R&D Program of China, Precision Medicine Program (No. 2017YFC0907700).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors have reviewed the manuscript and have no conflict of interest to declare. The manuscript has not been published or presented elsewhere in part or in entirety, and is not under consideration by another journal.
Ethical statements
This study was approved by the institutional ethics committee of Xuanwu Hospital, Capital Medical University and the Laboratory Animal Ethics Committee of Xuanwu Hospital. Written informed consent was obtained from the patients.
Rights and permissions
About this article
Cite this article
Lei, L., Chen, H., Lu, Y. et al. Unusual electrophysiological findings in a Chinese ALS 4 family with SETX-L389S mutation: a three-year follow-up. J Neurol 268, 1050–1058 (2021). https://doi.org/10.1007/s00415-020-10246-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-020-10246-2