Abstract
Objective
To describe 123I-FP-CIT (DAT scan) SPECT findings in progressive apraxia of speech (PAOS) patients and to compare those findings with progressive supranuclear palsy (PSP) and corticobasal syndrome (CBS).
Background
PAOS is a neurodegenerative syndrome in which patients present with apraxia of speech, a motor speech disorder affecting programming and planning of speech. Patients with PAOS predictably develop Parkinsonism. DAT scan is a neuroimaging tool that assesses the integrity of presynaptic dopamine transporters in striatum and is usually abnormal in PSP and CBS.
Methods
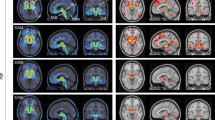
As part of an NIH-funded grant, we performed a DAT scan on 17 PAOS patients early in the disease course. DaTQUANT software was used to quantify uptake in the left and right caudate and anterior/posterior putamen, with striatum to background ratios (SBRs). The PAOS cohort was compared to 15 PSP and 8 CBS patients.
Results
Five PAOS patients (29%) showed abnormalities in at least one striatal region on DAT scan. When the five PAOS patients with abnormal DAT were compared to the PSP and CBS patients, the only difference observed was lower uptake in the posterior putamen in PSP (p = 0.03). There were no differences is putamen/caudate ratio or in symmetry of uptake, across all groups. There was also no difference in MDS-UPDRS-III scores between PAOS patients with and without abnormal DAT scans (p = 0.56).
Conclusions
Abnormal DAT scan is observed early in the disease course in approximately 30% of PAOS patients, with striatal abnormalities similar to those in PSP and CBS.


Similar content being viewed by others
References
McNeil MR, Robin DA, Schmidt RA (2009) Apraxia of speech: definition and differential diagnosis. Clin Manag Sens Speech Disord 2:249–267
Josephs KA et al (2014) The evolution of primary progressive apraxia of speech. Brain 137(10):2783–2795
Duffy JR (2006) Apraxia of speech in degenerative neurologic disease. Aphasiology 20(6):511–527
Josephs KA et al (2005) Atypical progressive supranuclear palsy underlying progressive apraxia of speech and nonfluent aphasia. Neurocase 11(4):283–296
Josephs KA et al (2012) Characterizing a neurodegenerative syndrome: primary progressive apraxia of speech. Brain 135(5):1522–1536
Josephs KA et al (2006) Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain 129(6):1385–1398
Whitwell JL et al (2019) An evaluation of the progressive supranuclear palsy speech/language variant. Mov Disord Clin Pract 6(6):452–461
Tetzloff KA et al (2018) Clinical and imaging progression over 10 years in a patient with primary progressive apraxia of speech and autopsy-confirmed corticobasal degeneration. Neurocase 24(2):111–120
Utianski RL et al (2018) Clinical progression in four cases of primary progressive apraxia of speech. Am J Speech Lang Pathol 27(4):1303–1318
Marshall VL et al (2009) Parkinson’s disease is overdiagnosed clinically at baseline in diagnostically uncertain cases: a 3-year European multicenter study with repeat [123I]FP-CIT SPECT. Mov Disord 24(4):500–508
Seifert KD, Wiener JI (2013) The impact of DaTscan on the diagnosis and management of movement disorders: a retrospective study. Am J Neurodegener Dis 2(1):29–34
Pirker W et al (2000) [123I]beta-CIT SPECT in multiple system atrophy, progressive supranuclear palsy, and corticobasal degeneration. Mov Disord 15(6):1158–1167
Klaffke S et al (2006) Dopamine transporters, D2 receptors, and glucose metabolism in corticobasal degeneration. Mov Disord 21(10):1724–1727
Im JH et al (2006) Differential patterns of dopamine transporter loss in the basal ganglia of progressive supranuclear palsy and Parkinson’s disease: analysis with [(123)I]IPT single photon emission computed tomography. J Neurol Sci 244(1–2):103–109
McKhann G et al (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34(7):939–944
McKeith IG et al (2005) Diagnosis and management of dementia with Lewy bodies: third report of the DLB consortium. Neurology 65(12):1863–1872
Neary D et al (1998) Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 51(6):1546–1554
Litvan I et al (1996) Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele–Richardson–Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology 47(1):1–9
Boeve BF, Lang AE, Litvan I (2003) Corticobasal degeneration and its relationship to progressive supranuclear palsy and frontotemporal dementia. Ann Neurol 54(5):S15–S19
Gilman S et al (2008) Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71(9):670–676
Brooks BR et al (2000) El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 1(5):293–299
Duffy JR, Strand EA, Josephs KA (2014) Motor speech disorders associated with primary progressive aphasia. Aphasiology 28(8–9):1004–1017
Nasreddine ZS et al (2005) The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53(4):695–699
Hoglinger GU et al (2017) Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov Disord 32(6):853–864
Whitwell JL et al (2011) Clinical correlates of white matter tract degeneration in progressive supranuclear palsy. Arch Neurol 68(6):753–760
Goetz CG et al (2008) Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 23(15):2129–2170
Kertesz A, Davidson W, Fox H (1997) Frontal behavioral inventory: diagnostic criteria for frontal lobe dementia. Can J Neurol Sci 24(1):29–36
Strauss SA (1998) An unusual case of wrongful pregnancy: liability of doctor resulting from misrepresentation. Med Law 17(1):7–11
Maltais DD et al (2020) Confirmation of (123)I-FP-CIT-SPECT (ioflupane) quantification methods in dementia with Lewy body and other neurodegenerative disorders. J Nucl Med 2020:119
Antonini A et al (2003) 123I-Ioflupane/SPECT binding to striatal dopamine transporter (DAT) uptake in patients with Parkinson’s disease, multiple system atrophy, and progressive supranuclear palsy. Neurol Sci 24(3):149–150
Badoud S et al (2016) Discriminating among degenerative parkinsonisms using advanced (123)I-ioflupane SPECT analyses. Neuroimage Clin 12:234–240
Iranzo A et al (2017) Dopamine transporter imaging deficit predicts early transition to synucleinopathy in idiopathic rapid eye movement sleep behavior disorder. Ann Neurol 82(3):419–428
Stephenson D et al (2019) The qualification of an enrichment biomarker for clinical trials targeting early stages of Parkinson’s disease. J Parkinsons Dis 9(3):553–563
Nicastro N, Burkhard PR, Garibotto V (2018) Scan without evidence of dopaminergic deficit (SWEDD) in degenerative parkinsonism and dementia with Lewy bodies: a prospective study. J Neurol Sci 385:17–21
Marek K et al (2014) Longitudinal follow-up of SWEDD subjects in the PRECEPT study. Neurology 82(20):1791–1797
Josephs KA et al (2013) Syndromes dominated by apraxia of speech show distinct characteristics from agrammatic PPA. Neurology 81(4):337–345
Utianski RL et al (2018) Prosodic and phonetic subtypes of primary progressive apraxia of speech. Brain Lang 184:54–65
Funding
This project is funded by NIH Grants R01 DC14942 and R01 DC12519.
Author information
Authors and Affiliations
Contributions
ZIS, JLW and KAJ have full access to all data, and take responsibility for the accuracy of the data analyses and interpretation. FA contributed to patient recruitment. All authors contributed to the study concept and design, discussed the data results, contributed to drafting of the manuscript and approved the final version for submission. KAJ is the guarantor of the paper.
Corresponding author
Ethics declarations
Conflicts of interest
No conflicts exist for any of the authors.
Rights and permissions
About this article
Cite this article
Seckin, Z.I., Whitwell, J.L., Utianski, R.L. et al. Ioflupane 123I (DAT scan) SPECT identifies dopamine receptor dysfunction early in the disease course in progressive apraxia of speech. J Neurol 267, 2603–2611 (2020). https://doi.org/10.1007/s00415-020-09883-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-020-09883-4