Abstract
Background/objective
Two functional networks are proposed as neuronal support for the complex processes of memory: the anterior temporal and the medial posterior systems. We examined the atrophy of hippocampus (HC) and of those areas constituting the two functional memory systems in multiple sclerosis (MS) patients with low disability.
Methods
Episodic memory (EM) was assessed in 88 relapsing MS patients and in 40 healthy controls using Wechsler Memory Scale III (Spanish adaptation). FreeSurfer software was used to calculate normalized volume of total cortex, grey matter, white matter, subcortical grey matter (thalamus and striatum), HC and both the anterior temporal (entorhinal, ventral temporopolar, lateral orbitofrontal, amygdala) and posterior medial systems (thalamus, parahippocampal, posterior cingulate, precuneus, lateral parietal and medial prefrontal). Linear regression analysis was used to identify predictors of memory performance.
Results
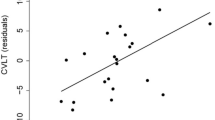
Total grey matter and cortex volumes correlated with all subtypes of EM, and the precuneus volume correlated with overall, immediate and delayed memories. Univariant regression analysis identified an association between the volumes of the posterior medial memory network regions and EM scores. The volume of the left precuneus area was the unique and independent predictor for all EM subtypes except for visual memory, for which left HC volume was also an independent predictor.
Conclusion
Left precuneus volume was the best predictor of memory in relapsing MS patients with low disability and mild deficits in EM.
Similar content being viewed by others
References
Amato MP, Zipoli V, Portaccio E (2008) Cognitive changes in multiple sclerosis. Expert Rev Neurother 8:1585–1596. https://doi.org/10.1586/14737175.8.10.1585
Saenz A, Bakchine S, Jonin PY et al (2015) Multiple sclerosis and verbal episodic memory: critical review of cognitive processes and their assessment. Rev Neurol (Paris) 171:624–645
Brissart H, Morele E, Baumann C et al (2012) Verbal episodic memory in 426 multiple sclerosis patients: impairment in encoding, retrieval or both? Neurol Sci 33:1117–1123. https://doi.org/10.1007/s10072-011-0915
Travis SG, Huang Y, Fujiwara E et al (2014) High field structural MRI reveals specific episodic memory correlates in the subfields of the hippocampus. Neuropsychologia 53:233–245. https://doi.org/10.1016/j.neuropsychologia.2013.11.016
Griffith HR, Pyzalski RW, Seidenberg M et al (2004) Memory relationships between MRI volumes and resting PET metabolism of medial temporal lobe structures. Epilepsy Behav 5:669–676
Geurts JJ, Bö L, Roosendaal SD et al (2007) Extensive hippocampal demyelination in multiple sclerosis. J Neuropathol Exp Neurol 66:819–827
Papadopoulos D, Dukes S, Patel R et al (2009) Substantial archaeocortical atrophy and neuronal loss in multiple sclerosis. Brain Pathol 19:238–253
Geurts JJ, Pouwels PJ, Uitdehaag BM et al (2005) Intracortical lesions in multiple sclerosis: improved detection with 3D double inversion recovery MR imaging. Radiology 263:254–260
Sicotte NL, Kern KC, Giesser BS et al (2008) Regional hippocampal atrophy in multiple sclerosis. Brain 131:1134–1141
Benedict RH, Ramasamy D, Munschauer F et al (2009) Memory impairment in multiple sclerosis: correlation with deep grey matter and mesial temporal atrophy. J Neurol Neurosurg Psychiatry 80:201–206
Longoni G, Rocca MA, Pagani E et al (2015) Deficits in memory and visuospatial learning correlate with regional hippocampal atrophy in MS. Brain Struct Funct 220:435–444
González Torre JA, Cruz-Gómez ÁJ, Belenguer A et al (2017) Hippocampal dysfunction is associated with memory impairment in multiple sclerosis: a volumetric and functional connectivity study. Mult Scler 23:1854–1863. https://doi.org/10.1177/1352458516688349
Ranganath C, Ritchey M (2012) Two cortical systems for memory-guided behavior. Nat Rev Neurosci 13:713–726. https://doi.org/10.1038/nrn3338
Ritchey M, Libby LA, Ranganath C (2015) Cortico-hippocampal systems involved in memory and cognition: the PMAT framework. Prog Brain Res 219:45–64. https://doi.org/10.1016/bs.pbr.2015.04.001
Sumowski JF, Leavitt VM, Rocca MA et al (2017) Mesial temporal lobe and subcortical grey matter volumes differentially predict memory across stages of multiple sclerosis. Mult Scler. https://doi.org/10.1177/1352458517708873
Polman C, Reingold S, Banwell B et al (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69:292–302
Wechsler D (2004) Wechsler Memory Scale, 3rd edn. TEA Ediciones, Madrid
Schmidt P, Gaser C, Arsic M et al (2012) An automated tool for detection of FLAIR-hyperintense white-matter lesions in multiple sclerosis. Neuroimage 59:3774–3783
Pareto D, Sastre-Garriga J, Aymerich FX et al (2016) Lesion filling effect in regional brain volume estimations: a study in multiple sclerosis patients with low lesion load. Neuroradiology 58:467. https://doi.org/10.1007/s00234-016-1654-5
Valverde S, Oliver A, Roura E et al (2015) Quantifying brain tissue volume in multiple sclerosis with automated lesion segmentation and filling. NeuroImage Clin 9:640–647. https://doi.org/10.1016/j.nicl.2015.10.012
Biberacher V, Schmidt P, Keshavan A et al (2016) Intra and interscanner variability of magnetic resonance imaging based volumetry in multiple sclerosis. NeuroImage 142:188–197. https://doi.org/10.1016/j.neuroimage.2016.07.035
Magon S, Gaetano L, Chakravarty MM et al (2014) White matter lesion filling improves the accuracy of cortical thickness measurements in multiple sclerosis patients: a longitudinal study. BMC Neurosci 15:106. https://doi.org/10.1186/1471-2202-15-106
Fischl B, Dale AM (2000) Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 97:11050–11055
Cavanna AE, Trimble MR (2006) The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129:564–583. https://doi.org/10.1093/brain/aw1004
Utevsky AV, Smith DV, Huettel SA (2014) Precuneus is a functional core of the default-mode network. J Neurosci 34:932–940. https://doi.org/10.1523/JNEUROSCI.4227-13.2014
Schmidt D, Krause BJ, Mottaghy FM et al (2002) Brain systems engaged in encoding and retrieval of word-pair associates independent of their imagery content or presentation modalities. Neuropsychologia 40:457–470. https://doi.org/10.1016/S0028-3932(01)00102-6
Henson RN, Rugg MD, Shallice T et al (1999) Recollection and familiarity in recognition memory: an event-related functional magnetic resonance imaging study. J Neurosci 19:3962–3972
Lundstrom BN, Ingvar M, Petersson KM (2005) The role of precuneus and left inferior frontal cortex during source memory episodic retrieval. Neuroimage 27:824–834. https://doi.org/10.1016/j.neuroimage.2005.05.008
Peters J, Daum I, Gizewski E et al (2009) Associations evoked during memory encoding recruit the context-network. Hippocampus 19:141–151. https://doi.org/10.1002/hipo.20490
Dörfel D, Werner A, Scheafer M et al (2009) Distinct brain networks in recognition memory share a defined region in the precuneus. Eur J Neurosci 30:1947–1959. https://doi.org/10.1111/j.1460-9568.2009.06973.x
Eustache F, Piolino P, Giffard B et al (2004) “In the course of time”: a PET study of the cerebral substrates of autobiographical amnesia in Alzheimer’s disease. Brain 127:1549–1560. https://doi.org/10.1093/brain/awh166
Bailly M, Destrieux C, Hommet C et al (2015) Precuneus and cingulate cortex atrophy and hypometabolism in patients with Alzheimer’s disease and mild cognitive impairment: MRI and F-18-FDG PET quantitative analysis using freesurfer. Biomed Res Int. https://doi.org/10.1155/2015/583931
Klaassens BL, van Gerven JMA, van der Grond J et al (2017) Diminished posterior precuneus connectivity with the default mode network differentiates normal aging from Alzheimer’s disease. Front Aging Neurosci 9:97. https://doi.org/10.3389/fnagi.2017.00097
Freton M, Lemogne C, Bergouignan L et al (2014) The eye of the self: precuneus volume and visual perspective during autobiographical memory retrieval. Brain Struct Funct 219:959–968. https://doi.org/10.1007/s00429-013-0546-2
Burgess N, Maguire EA, O’Keefe J (2002) The human hippocampus and spatial and episodic memory. Neuron 35:625–641
Acknowledgements
The authors are grateful to MS patients and HC for their participation.
Funding
This research was supported by Novartis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Authors declare not having conflicts of interest with respect to this work. We report all possible disclosures to be thorough. Y. A. had received speakers’ honoraria from Novartis, Biogen Idec, Teva and Merck Serono; serves as a consultant and scientific advisory board for some Pharmaceutical Industries (Teva, Novartis, Biogen Idec, Sanofi Genzyme and Merck Serono). M. C. received support to investigate from Novartis and Teva.
Ethical standard
All procedures performed in this study involving human participants were carried out respecting the principles established in the 1964 Helsinki Declaration and its later amendments, the requirements by the Spanish legislation on biomedical investigation and protection of personal data.
Rights and permissions
About this article
Cite this article
Aladro, Y., López-Alvarez, L., Sánchez-Reyes, J.M. et al. Relationship between episodic memory and volume of the brain regions of two functional cortical memory systems in multiple sclerosis. J Neurol 265, 2182–2189 (2018). https://doi.org/10.1007/s00415-018-8965-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-018-8965-x