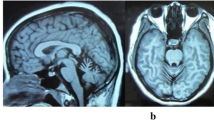
Abstract
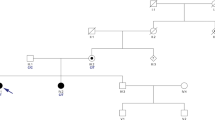
We report three affected members, a mother and her two children, of a non-consanguineous Irish family who presented with a suspected autosomal dominant spinocerebellar ataxia characterized by early motor delay, poor coordination, gait ataxia, and dysarthria. Whole exome sequencing identified a novel missense variant (c.106C>T; p.[Arg36Cys]) in the suppressor domain of type 1 inositol 1,4,5-trisphosphate receptor gene (ITPR1) as the cause of the disorder, resulting in a molecular diagnosis of spinocerebellar ataxia type 29. In the absence of grandparental DNA, microsatellite genotyping of healthy family members was used to confirm the de novo status of the ITPR1 variant in the affected mother, which supported pathogenicity. The Arg36Cys variant exhibited a significantly higher IP3-binding affinity than wild-type (WT) ITPR1 and drastically changed the property of the intracellular Ca2+ signal from a transient to a sigmoidal pattern, supporting a gain-of-function disease mechanism. To date, ITPR1 mutation has been associated with a loss-of-function effect, likely due to reduced Ca2+ release. This is the first gain-of-function mechanism to be associated with ITPR1-related SCA29, providing novel insights into how enhanced Ca2+ release can also contribute to the pathogenesis of this neurological disorder.




Similar content being viewed by others
References
Fogel BL, Lee H, Deignan JL et al (2014) Exome sequencing in the clinical diagnosis of sporadic or familial cerebellar ataxia. JAMA Neurol 71:1237–1246
Ohba C, Osaka H, Iai M et al (2013) Diagnostic utility of whole exome sequencing in patients showing cerebellar and/or vermis atrophy in childhood. Neurogenetics 14:225–232
Huang L, Chardon JW, Carter MT et al (2012) Missense mutations in ITPR1 cause autosomal dominant congenital nonprogressive spinocerebellar ataxia. Orphanet J Rare Dis 7:67
Sasaki M, Ohba C, Iai M et al (2015) Sporadic infantile-onset spinocerebellar ataxia caused by missense mutations of the inositol 1,4,5-triphosphate receptor type 1 gene. J Neurol 262:1278–1284
Barresi S, Niceta M, Alfieri P et al (2016) Mutations in the IRBIT domain of ITPR1 are a frequent cause of autosomal dominant nonprogressive congenital ataxia. Clin Genet 91:86–91
van de Leemput J, Chandran J, Knight MA et al (2007) Deletion at ITPR1 underlies ataxia in mice and spinocerebellar ataxia 15 in humans. PLoS Genet 3:e108
Hara K, Shiga A, Nozaki H et al (2008) Total deletion and a missense mutation of ITPR1 in Japanese SCA15 families. Neurology 71:547–551
Marelli C, van de Leemput J, Johnson JO et al (2011) SCA15 due to large ITPR1 deletions in a cohort of 333 white families with dominant ataxia. Arch Neurol 68:637–643
Novak MJ, Sweeney MG, Li A et al (2010) An ITPR1 gene deletion causes spinocerebellar ataxia 15/16: a genetic, clinical and radiological description. Mov Disord 25:2176–2182
Iwaki A, Kawano Y, Miura S et al (2008) Heterozygous deletion of ITPR1, but not SUMF1, in spinocerebellar ataxia type 16. J Med Genet 45:32–35
Di Gregorio E, Orsi L, Godani M et al (2010) Two Italian families with ITPR1 gene deletion presenting a broader phenotype of SCA15. Cerebellum 9:115–123
Obayashi M, Ishikawa K, Izumi Y et al (2012) Prevalence of inositol 1,4,5-triphosphate receptor type 1 gene deletion, the mutation for spinocerebellar ataxia type 15, in Japan screened by gene dosage. J Hum Genet 57:202–206
Gerber S, Alzayady KJ, Burglen L et al (2016) Recessive and dominant de novo ITPR1 mutations cause Gillespie syndrome. Am J Hum Genet 98:971–980
McEntagart M, Williamson KA, Rainger JK et al (2016) A restricted repertoire of de novo mutations in ITPR1 cause Gillespie syndrome with evidence for dominant-negative effect. Am J Hum Genet 98:981–992
Berridge MJ (1993) Inositol trisphosphate and calcium signaling. Nature 361:315–325
Mikoshiba K (2007) IP3 receptor/Ca2+ channel: from discovery to new signaling concepts. J Neurochem 102:1426–1446
Casey JP, Støve SI, McGorrian C et al (2015) NAA10 mutation causing a novel intellectual disability syndrome with Long QT due to N-terminal acetyltransferase impairment. Sci Rep 5:16022
Purcell S, Neale B, Todd-Brown K et al (2007) PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81:559–575
Yamada N, Makino Y, Clark RA et al (1994) Human inositol 1,4,5-trisphosphate type-1 receptor, InsP3R1: structure, function, regulation of expression and chromosomal localization. Biochem J 302:781–790
Yoshikawa F, Uchiyama T, Iwasaki H et al (1999) High efficient expression of the functional ligand binding site of the inositol 1,4,5-triphosphate receptor in Escherichia coli. Biochem Biophys Res Commun 257:792–797
Yamazaki H, Nozaki H, Onodera O, Michikawa T, Nishizawa M, Mikoshiba K (2011) Functional characterization of the P1059L mutation in the inositol 1,4,5-trisphosphate receptor type 1 identified in a Japanese SCA15 family. Biochem Biophys Res Commun 410:754–758
Yoshikawa F, Morita M, Monkawa T, Michikawa T, Furuichi T, Mikoshiba K (1996) Mutational analysis of the ligand binding site of the inositol 1,4,5-trisphosphate receptor. J Biol Chem 271:18277–18284
Uchida K, Miyauchi H, Furuichi T, Michikawa T, Mikoshiba K (2003) Critical regions for activation gating of the inositol 1,4,5-trisphosphate receptor. J Biol Chem 278:16551–16560
Sugawara H, Kurosaki M, Takata M, Kurosaki T (1997) Genetic evidence for involvement of type 1, type 2 and type 3 inositol 1,4,5-trisphosphate receptors in signal transduction through the B-cell antigen receptor. EMBO J 16:3078–3088
Miyakawa T, Maeda A, Yamazawa T, Hirose K, Kurosaki T, Iino M (1999) Encoding of Ca2+ signals by differential expression of IP3 receptor subtypes. EMBO J 18:1303–1308
Dudding TE, Friend K, Schofield PW, Lee S, Wilkinson IA, Richards RI (2004) Autosomal dominant congenital non-progressive ataxia overlaps with the SCA15 locus. Neurology 63:2288–2292
Synofzik M, Beetz C, Bauer C et al (2011) Spinocerebellar ataxia type 15: diagnostic assessment, frequency, and phenotypic features. J Med Genet 48:407–412
Kattah JC, Kolsky MP, Guy J, O’Doherty D (1983) Primary position vertical nystagmus and cerebellar ataxia. Arch Neurol 40:310–314
Steinlin M, Zangger B, Boltshauser E (1998) Non-progressive congenital ataxia with or without cerebellar hypoplasia: a review of 34 subjects. Dev Med Child Neurol 40:148–154
Gonzaga-Jauregui C, Harel T, Gambin T et al (2015) Exome sequence analysis suggests that genetic burden contributes to phenotypic variability and complex neuropathy. Cell Rep 12:1169–1183
Bosanac I, Yamazaki H, Matsu-ura T, Michikawa T, Mikoshiba K, Ikura M (2005) Crystal structure of the ligand binding suppressor domain of type 1 inositol 1,4,5-trisphosphate receptor. Mol Cell 17:193–203
Rossi AM, Riley AM, Tovey SC et al (2009) Synthetic partial agonists reveal key steps in IP3 receptor activation. Nat Chem Biol 5:631–639
Lin CC, Baek K, Lu Z (2011) Apo and InsP3-bound crystal structures of the ligand-binding domain of an InsP3 receptor. Nat Struct Mol Biol 18:1172–1174
Liu J, Tang TS, Tu H, Nelson O, Herndon E, Huynh DP, Pulst SM, Bezprozvanny I (2009) Deranged calcium signaling and neurodegeneration in spinocerebellar ataxia type 2. J Neurosci 29:9148–9162
Kasumu A, Liang X, Egorova P, Vorontsova D, Bezprozvanny I (2012) Chronic suppression of inositol 1, 4,5-triphosphate receptor-mediated calcium signaling in cerebellar Purkinje cells alleviates pathological phenotype in spinocerebellar ataxia 2 mice. J Neurosci 32:12786–12796
Egorova P, Zakharova O, Vlasova O, Bezprozvanny I (2016) In vivo analysis of cerebellar Purkinje cell activity in SCA2 transgenic mouse model. J Neurophysiol 115:2840–2851
Tu JC, Xiao B, Yuan JP et al (1998) Homer binds a novel proline rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron 21:717–726
Kasri NN, Holmes AM, Bultynck G et al (2004) Regulation of InsP3 receptor activity by neuronal Ca2+-binding proteins. EMBO J 23:312–321
Bezprozvanny I (2011) Role of inositol 1,4,5-trishosphate receptors in pathogenesis of Huntington’s disease and spinocerebellar ataxias. Neurochem Res 36(7):1186–1197
Acknowledgements
We sincerely thank the family involved for their significant contribution to research and the use of genetic samples and clinical information. This work was supported by a Medical Research Charities Group Grant (to S.A.L. and J.P.C.) from the Health Research Board (MRCG/2013/02) and the Children’s Fund for Health, The Fundraising Office for Temple Street Children’s University Hospital, Dublin, Ireland (MRCG/2013/02). Jillian Casey is supported by a Medical Research Charities Group Grant (MRCG/2013/02). Functional studies were supported by Grant-in-Aid for Scientific Research (S) Grant Number 25221002 (to K.M) and Grant-in-Aid for Scientific Research (C) Grant Number 15K06761 (to C.H). We are grateful to the Support Unit for Bio-Material Analysis, RIKEN BSI Research Resources Center, for technical help with plasmid sequence analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Ethical standards
This study was approved by the appropriate ethics committee and has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Casey, J.P., Hirouchi, T., Hisatsune, C. et al. A novel gain-of-function mutation in the ITPR1 suppressor domain causes spinocerebellar ataxia with altered Ca2+ signal patterns. J Neurol 264, 1444–1453 (2017). https://doi.org/10.1007/s00415-017-8545-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-017-8545-5