Abstract
Sleep disturbances are common in multiple sclerosis (MS), but its impact on cognition and functional connectivity (FC) of the hippocampus and thalamus is unknown. Therefore, we investigated the relationship between sleep disturbances, cognitive functioning and resting-state (RS) FC of the hippocampus and thalamus in MS. 71 MS patients and 40 healthy controls underwent neuropsychological testing and filled out self-report questionnaires (anxiety, depression, fatigue, and subjective cognitive problems). Sleep disturbances were assed with the five-item version of the Athens Insomnia Scale. Hippocampal and thalamic volume and RS FC of these regions were determined. Twenty-three patients were categorized as sleep disturbed and 48 as normal sleeping. No differences were found between disturbed and normal sleeping patients concerning cognition and structural MRI. Sleep disturbed patients reported more subjective cognitive problems, and displayed decreased FC between the thalamus and middle and superior frontal gyrus, inferior frontal operculum, anterior cingulate cortex, inferior parietal gyrus, precuneus, and angular gyrus compared to normal sleeping patients. We conclude that sleep disturbances in MS are not (directly) related to objective cognitive functioning, but rather to subjective cognitive problems. In addition, sleep disturbances in MS seem to coincide with a specific pattern of decreased thalamic FC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Up to 65 % of multiple sclerosis (MS) patients suffer from cognitive problems [1], resulting in a reduced quality of life [2]. Several factors are thought to negatively influence cognition in MS patients, such as depression [3], fatigue [4], and sleep disturbances [5]. Approximately 50 % of the patients with MS suffer from sleep disturbances (e.g., insomnia or sleep-disordered breathing) [6].
In healthy controls (HCs), proper sleep is important for memory consolidation [7] and sleep deprivation has been related to impaired functioning in various cognitive domains [8]. The literature on sleep disturbances and cognition in MS is scarce. One study showed an association between sleep disturbances and a decline in sustained attention [9], whereas another study related reduced sleep efficiency to problems with information processing and executive function [5].
On functional (f) magnetic resonance imaging (MRI), the effects of sleep disturbances can be seen as hypo-activation in medial and inferior prefrontal areas in subjects with insomnia compared to HCs during a cognitive task, which returned to normal values after sleep therapy [10]. In addition, shallow sleep has been related to reduced hippocampal activation [11], and the thalamus showed decreased functional connectivity (FC) in sleep deprived HCs [12].
In MS, damage to the hippocampus and thalamus (e.g., lesions and atrophy) is associated with worse cognition [13, 14]. In HCs, both regions can be related to sleep and cognition. In the present study, we investigated sleep disturbances in MS in relation to cognitive functioning and resting-state (RS) FC of the hippocampus and thalamus. We hypothesize that sleep problems negatively influence cognition and can be related to FC alterations of the hippocampus and thalamus.
Materials and methods
Participants
All patients (n = 71; 47 female; mean disease duration 11.0 years) were diagnosed with clinically definite MS according to the revised McDonald criteria [15]. On the day of scanning, disease severity was measured using a questionnaire based on the expanded disability status scale [16]. Age- and sex matched HCs (n = 40; 26 female) were included. Subjects included in this study are partly overlapping with a previously reported fMRI study [17]. Exclusion criteria were the presence or history of psychiatric or neurological diseases (for patients: other than MS) and contra-indications for MRI. All participants gave written informed consent prior to participation. The institutional ethical review board approved the study protocol and it has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Sleep disturbances
The Athens Insomnia Scale (AIS) is a self-report questionnaire, validated in HCs, and was used to measure sleep disturbances [18]. This questionnaire includes eight items on which a score ranging from zero to three points (no to severe problems) can be obtained for each item. As the eight-item version of the AIS includes three items that can reflect MS symptoms independent from sleep problems (e.g., fatigue during the day), the five-item version of the AIS was used. This version assesses difficulty with sleep quality and quantity, and includes the following items: sleep induction time, awakening during the night, final awakening earlier than desired, total sleep duration, and overall quality of sleep. We categorized patients as ‘sleep disturbed’ if they scored at least three points (which is the median score of patients) with a prerequisite that at least one item should be scored ≥2 (moderate to severe problems). Otherwise, patients were categorized as ‘normal sleeping’.
Neuropsychological evaluation
All subjects underwent an extensive neuropsychological test battery, consisting of the following tests:
-
The Dutch equivalent of the California Verbal Learning Test, the Verbale Leer- en Geheugen Taak (VLGT) [19], to assess verbal learning and memory;
-
Letter Digit Substitution Task (LDST; an adaptation of the symbol digit modalities test) [20], to assess information processing speed;
-
Location Learning Test (LLT) [21], to assess visuospatial memory;
-
Digit Span forward and backward, subtests of the Wechsler Adult Intelligence Scale [22], to assess short term and working memory, respectively;
-
World List Generation, including three categories: animals, professions, and m-words (1 min per subtest), to asses verbal fluency [23].
All test scores were converted into Z-scores relative to HCs. For each subject, all Z-scores were averaged to obtain an average cognition score. Patients were categorized as cognitively impaired if they scored at least two standard deviations below that of HCs on at least two out of five tests. Otherwise, patients were classified as cognitively preserved.
Symptoms of depression, anxiety, fatigue, and subjective cognitive problems were assessed using the Hospital Anxiety and Depression Scale (HADS) [24], the Checklist of Individual Strength (CIS-20) [25], and the Cognitive Function Scale (CFS) for subjective cognitive functioning from the Medical Outcomes study [26].
MRI acquisition
All subjects were scanned on 1.5T (Siemens Sonata, Erlangen, Germany). Structural MRI consisted of 3DT1-weighted magnetization prepared rapid acquisition gradient-echo (MPRAGE) images and turbo spin-echo proton density (PD)/T2-weighted images. RS fMRI was performed to calculate FC.
Structural MRI analysis
All imaging processing steps were performed in FSL 5.0 (FMRIB’s Software Library, http://www.fmrib.ox.ac.uk/fsl). Gray matter (GM) and white matter (WM) volumes were obtained using the MPRAGE images and SienaX [27]. FIRST [28] was used to measure the volume of the hippocampus and thalamus. All volumetric measures were normalized for head size. White matter lesions were manually marked and outlined on the PD/T2-weighted scan using a local threshold technique.
Functional MRI analysis
See the online resources for a detailed description of the FC analysis. In brief, the Automated Anatomical Labelling (AAL) atlas [29] was registered to each subject’s fMRI scan in native space to which subcortical structures were added. This novel atlas, containing 92 regions, was masked for GM, and from each atlas region, the average time series was obtained. FC was calculated between the hippocampus and thalamus (bilateral) and all other brain areas using synchronization likelihood (SL) [30] in BrainWave (http://home.kpn.nl/stam7883/index.html). SL is a measure for linear and nonlinear correlations and ranges from zero to one, and has been previously applied in MS [14].
Statistical analysis
All analyses were performed using the Statistical Package for the Social Sciences (SPSS, Chicago, IL) version 20. All statistical analyses were performed on group level. Normality of the data was tested with the Kolmogorov–Smirnov test. T2 lesion load was log-transformed, and SL values were inverted (1/SL) to achieve normality; all the other non-normally distributed variables were tested with the Mann–Whitney U test. General linear models were used to assess group differences. Univariate and multivariate regression analyses were used to predict AIS score and overall cognitive functioning in MS. A p value of 0.05 was considered statistically significant for demographic, behavioural, and structural MRI data and for the regression analyses. To be more conservative concerning the multiple comparisons in the FC analysis, a significance level of 0.01 was used.
Correlations between volumes of the left and right hippocampus and left and right thalamus were 0.73 and 0.95, respectively. Therefore, volumes of the left and right hippocampus and left and right thalamus were added up and treated as single measures to limit the number of variables in the analyses. In the FC analysis, the left and right hippocampus and thalamus were analysed separately, to be more specific.
Results
Demographics, neuropsychological evaluation, and MRI in MS vs. HCs
See Supplementary Table 1 for detailed information concerning the demographics and cognitive test scores of patients and HCs. Patients and HCs did not differ significantly with respect to age (mean age patients 45.7 years; mean age HCs 44.0 years; p = 0.338), sex (p = 0.898), and educational level (median educational level HCs and patients: 6.00; p = 0.627). Patients show higher levels of anxiety (p = 0.001), depression (p < 0.001), fatigue (p < 0.001), subjective cognitive problems (p < 0.001), and sleep disturbances (p = 0.002) compared to HCs. No significant relationship was found between AIS score, fatigue, and subjective cognitive problems in all MS patients. In HCs, higher AIS score was positively correlated with fatigue (Spearman’s ρ = 0.57, p < 0.001).
Patients performed worse on all cognitive tests compared to HCs. In MS, no significant relationship was found between AIS score and overall objective cognitive functioning or individual neuropsychological test scores. However, in HCs, higher AIS score was correlated with worse LDST performance (Spearman’s ρ = −0.35, p = 0.026).
MS patients had reduced normalized GM volume (NGMV; p = 0.001), normalized WM volume (NWMV; p < 0.001), normalized hippocampal volume (NHV; p < 0.001), and normalized thalamic volume (NTV; p < 0.001) compared to HCs. The hippocampus and thalamus showed increased FC in patients compared to HCs (see Supplementary Table 2).
Sleep disturbances in MS
Twenty-three MS patients (32 %) were classified as having sleep disturbances (see Table 1). Sleep disturbed patients reported higher levels of subjective cognitive problems (p = 0.023) compared to patients with normal sleep.
Cognition
No differences were found between disturbed and normal sleeping patients with regard to cognitive functioning (see Table 2). Twelve sleep disturbed patients (52 %) were categorized as cognitively impaired versus 14 (29 %) normal sleeping patients (p = 0.060).
Structural MRI
Disturbed and normal sleeping patients did not differ regarding structural imaging measures (see Table 3).
Functional connectivity
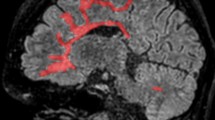
Table 4 displays functional connections that differed between disturbed and normal sleeping patients. Decreased FC was observed in sleep disturbed patients compared to normal sleeping patients between the thalamus and several cortical regions (see Fig. 1). None of the thalamic connections were increased. No differences in hippocampal FC were detected.
Regions displaying decreased functional connectivity with the thalamus in sleep disturbed compared to normal sleeping patients. For illustrative purposes, the atlas was registered to MNI standard space (1 mm) and brain regions were indicated by different colours. The upper panel (a) displays all connections of the left thalamus that showed decreased functional connectivity in sleep disturbed patients compared to normal sleeping patients. In the lower panel (b), all connections that showed decreased functional connectivity of the right thalamus in sleep disturbed patients compared to normal sleeping patients are visualized L left, R right
Predicting AIS score and cognition in MS
To obtain the most important predictors for AIS score in MS, the relationship between those FC measures that differed between disturbed and normal sleeping patients (1/SL), structural brain measures (NGMV, NWMV, NHV, NTV, and T2 lesion load), subjective cognitive problems (confounding variable), and AIS score were assessed using univariate regression analyses. The predictors that survived the univariate regression analyses were: NHV (adj. R 2 = 0.06, F = 5.44, β = 0.27, p = 0.023), FC between the left thalamus and left anterior cingulate cortex (adj. R 2 = 0.05, F = 4.36, β = 0.24, p = 0.040), FC between the right thalamus and left inferior parietal gyrus (adj. R 2 = 0.12, F = 9.21, β = 0.34, p = 0.003), left precuneus (adj. R 2 = 0.07, F = 6.56, β = 0.30, p = 0.013), left angular gyrus (adj. R 2 = 0.05, F = 4.84, β = 0.26, p = 0.031), right inferior frontal operculum (adj. R 2 = 0.13, F = 9.91, β = 0.35, p = 0.002), and right superior frontal gyrus (adj. R 2 = 0.08, F = 7.46, β = 0.31, p = 0.008). Multivariate backward regression analysis revealed that 16.3 % of variance in AIS score (F = 7.83, p = 0.001) could be explained by FC (1/SL) between the right thalamus and right inferior frontal operculum (β = 0.34, p = 0.003) and NHV (β = 0.25, p = 0.026).
Subsequently, univariate regression analyses were performed to identify the most important predictors for the overall cognitive functioning in MS, including the following variables: age, sex, educational level, subjective cognitive problems, NGMV, NWMV, NHV, NTV, and T2 lesions load. The predictors that survived the univariate regression analyses were: educational level (adj. R 2 = 0.10, F = 8.40, β = 0.33, p = 0.005), NGMV (adj. R 2 = 0.07, F = 6.46, β = 0.29, p = 0.013), NWMV (adj. R 2 = 0.08, F = 7.06, β = 0.31, p = 0.010), NHV (adj. R 2 = 0.10, F = 8.59, β = 0.33, p = 0.005), and NTV (adj. R 2 = 0.12, F = 10.53, β = 0.36, p = 0.002). A multivariate backward regression analysis demonstrated that 27.4 % of variance in the overall cognitive functioning (F = 9.67, p < 0.001) could be explained by the level of education (β = 0.42, p < 0.001), NHV (β = 0.29, p = 0.016), and NGMV (β = 0.24, p = 0.040). Subsequently, AIS score was entered into the model as a second step after the aforementioned predictors. Entering AIS score did not result in an increase in explained variance (β = −0.10, p = 0.362).
Discussion
Sleep disturbances and their effects on cognitive functioning and RS FC of the hippocampus and thalamus in MS patients were investigated. In our sample, 32 % of the patients were classified as having sleep disturbances. These patients had similar cognitive profiles compared to normal sleeping patients. Interestingly, decreased FC between the thalamus and the anterior cingulate cortex, precuneus, superior frontal gyrus, middle frontal gyrus, inferior frontal operculum, inferior parietal gyrus, and angular gyrus was found in patients with sleep disturbances compared to normal sleeping MS patients.
Contrary to our hypothesis, we did not observe a difference in objective cognitive functioning between patients with and without sleep disturbances that had an equal level of education and similar structural MRI measures. In our HCs, only information processing speed was negatively correlated with AIS score with a minor remark that our sample had limited variation in AIS score (6/40 HCs were defined as sleep disturbed).
In line with the literature, we found that sleep disturbed patients reported increased subjective cognitive problems. This was previously found in a large sample of 5171 HCs, in which the relationship between sleep disturbances and subjective cognitive functioning was stronger than that with objective cognitive functioning [31].
Although the literature is scarce, it was previously shown that subjective sleep problems in MS patients (with unknown disease duration) could be related to a decline in sustained attention during sequential sessions of a working memory task [9], whereas another study related poor sleep efficiency (measured using polysomnography and a multi-sleep latency test) to worse global cognitive performance (especially executive function and information processing) [5]. In the latter study, 32 patients (mean disease duration 7.5 years) were included and all treated with natalizumab. The difference in patient characteristics might explain the opposite findings since patients in the current study had an average disease duration of 10 years and varied concerning medication. Differences in the sleep measurements (i.e., questionnaires versus polysomnography) and samples might explain the discrepancy in results. Unfortunately, the previous studies did not include MRI to investigate the underlying brain mechanisms of sleep problems.
With regard to FC, patients with MS displayed exclusively increased FC in 22 hippocampal and thalamic connections relative to HCs, of which seven were related to the overall cognitive functioning (data not shown). With regard to sleep, patients with sleep disturbances showed decreased FC of thalamic connections compared to normal sleeping patients. In addition, the most important predictors for sleep disturbances in MS were: reduced FC between the right thalamus and right frontal operculum and larger hippocampal volume. The latter observation might be explained by the use of a self-report questionnaire to assess sleep disturbances. Although the link between self-report questionnaires and hippocampal volume has not been investigated to our knowledge, a previous study in MS found a similar relationship between larger hippocampal volume and higher levels of self-reported cognitive problems [32].
To our knowledge, this is the first study that investigated sleep disturbances in MS and its association with FC changes. Studies in HCs showed a link between decreased FC and sleep disturbances. For example, after sleep deprivation, decreased FC can be observed in the default mode network [33] and the thalamus [12]. Decreased FC between the thalamus and other regions, such as the superior frontal gyrus, gyrus rectus, precentral gyrus, postcentral gyrus, middle temporal gyrus, and anterior occipital lobe, was also found to be related to daytime sleepiness in an epidemiological study [34]. In the current study, we observed a decrease in FC of the thalamus in sleep disturbed patients, especially in connections between the thalamus and frontal areas (superior and middle frontal gyrus and inferior frontal operculum). This observation is in line with findings in HCs with sleep disturbances.
Thalamic connections that are affected when having sleep disturbances are different from the thalamic connections that are hampered when having cognitive disturbances in MS. In addition, a decrease in connectivity is seen for the sleep disturbed patients, while in relation to cognitive impairment, increased connectivity is mostly reported [14]. Patterns of decreased thalamocortical FC have also been observed in sleep deprived HCs [12]. Although it is not completely elucidated what the underlying mechanism is, in sleep deprived HCs, it was previously suggested to be a result of a decrease in brain metabolism (especially in frontal regions and the thalamus) as measured with positron emission tomography [35], possibly resulting in less synchronized firing of neurons. Our study suggests that a lack of sleep is related to highly specific changes in thalamic-cortico connectivity, which is not directly related to cognitive performance in MS patients.
One possible explanation for the absent relationship between sleep disturbances and cognitive performance might be that patients with severe sleep disturbances were not included in this sample, as subjects were not recruited based on their sleeping behavior. It can be hypothesized that severely sleep disturbed MS patients will be more similar to sleep deprived HCs, and perhaps do show impaired cognition. In our sample, the percentage of patients with sleep disturbances (32 %) was lower than reported previously (~50 %) [6]. The different numbers might be explained by the use of different self-report questionnaires. In the present study, we used the AIS which is a self-report questionnaire that has been validated in HCs [18]. Although it has not been validated in MS, it has been administered in other diseases, such as Alzheimer’s disease [36], which warrants its use in MS. Furthermore, the internal validity of the questionnaire in the present sample (Cronbach’s alpha) for MS patients and HCs was 0.70 and 0.74, respectively. The included items of the AIS assess problems with quality and quantity of sleep, and are not specific for the type of sleep problems that can be found due to MS (e.g., spasticity, sleep apnea, or pain). As no definition has been previously published for the five items version of the AIS, we defined sleep disturbances as scoring at least three points (i.e., median score of patients) with the prerequisite of scoring moderate to severe on at least one item, thereby aiming to be a bit more conservative than using, for instance, a median split approach. A previous study has shown that objective measures of sleep disturbances, such as obstructive sleep apnea, can be related to cognitive dysfunction in MS [37]. Hence, objective measures to quantify sleep disturbances, such as polysomnography, might give a more precise reflection than a self-report questionnaire of the sleep deficits being present. However, we do not expect that if we would have included objective measures of sleep disturbances, patients would have been categorized entirely different.
While FC changes in sleep disturbed MS patients follow a similar pattern compared to changes in connectivity in sleep deprived HCs, it might well be that the effect on cognitive performance is absent due to brain damage caused by MS. Our results suggest that educational level, hippocampal volume, and GM volume can predict overall cognitive functioning. Adding AIS score into the model did not improve the prediction of overall cognitive functioning. Hence, we hypothesize that the severity of structural brain damage in MS patients might be of more influence on cognition than the presence of sleep disturbances. That is, cortical and subcortical GM pathology, but also WM abnormalities, has been linked to impaired cognition in MS. The (widespread) structural brain abnormalities might limit the additional effect of sleep disturbances on cognition. It would be interesting to investigate an early cohort of patients with relatively mild brain pathology to see if sleep disturbances in that stage of the disease do (still) explain part of the cognitive deficits.
In summary, sleep disturbances, as measured with the AIS, in MS do not directly relate to objective cognitive functioning, but rather to subjective cognitive problems. The distinct FC pattern of the thalamus of sleep disturbed MS patients should be investigated in more depth to understand the complex interplay between sleep, cognition, and brain pathology in MS.
References
Rao SM, Leo GJ, Bernardin L, Unverzagt F (1991) Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction. Neurology 41:685–691
Mitchell AJ, ito-Leon J, Gonzalez JM, Rivera-Navarro J (2005) Quality of life and its assessment in multiple sclerosis: integrating physical and psychological components of wellbeing. Lancet Neurol 4:556–566
Arnett PA, Barwick FH, Beeney JE (2008) Depression in multiple sclerosis: review and theoretical proposal. J Int Neuropsychol Soc 14:691–724
Krupp LB, Elkins LE (2000) Fatigue and declines in cognitive functioning in multiple sclerosis. Neurology 55:934–939
Sater RA, Gudesblatt M, Kresa-Reahl K, Brandes DW, Sater PA (2015) The relationship between objective parameters of sleep and measures of fatigue, depression, and cognition in multiple sclerosis. Multiple Scler J Exp Transl Clin 1:1–8
Bamer AM, Johnson KL, Amtmann D, Kraft GH (2008) Prevalence of sleep problems in individuals with multiple sclerosis. Mult Scler 14:1127–1130
Walker MP (2009) The role of sleep in cognition and emotion. Ann N Y Acad Sci 1156:168–197
Lo JC, Groeger JA, Santhi N, Arbon EL, Lazar AS, Hasan S, Von SM, Archer SN, Dijk DJ (2012) Effects of partial and acute total sleep deprivation on performance across cognitive domains, individuals and circadian phase. PLoS One 7:e45987
Lehmann P, Eling P, Kastrup A, Grothues O, Hildebrandt H (2013) Self-reported sleep problems, but not fatigue, lead to decline in sustained attention in MS patients. Mult Scler 19:490–497
Altena E, van der Werf YD, Sanz-Arigita EJ, Voorn TA, Rombouts SA, Kuijer JP, Van Someren EJ (2008) Prefrontal hypoactivation and recovery in insomnia. Sleep 31:1271–1276
van der Werf YD, Altena E, Schoonheim MM, Sanz-Arigita EJ, Vis JC, De RW, Van Someren EJ (2009) Sleep benefits subsequent hippocampal functioning. Nat Neurosci 12:122–123
Shao Y, Wang L, Ye E, Jin X, Ni W, Yang Y, Wen B, Hu D, Yang Z (2013) Decreased thalamocortical functional connectivity after 36 hours of total sleep deprivation: evidence from resting state FMRI. PLoS One 8:e78830
Geurts JJ, Bo L, Roosendaal SD, Hazes T, Daniels R, Barkhof F, Witter MP, Huitinga I, van der Valk P (2007) Extensive hippocampal demyelination in multiple sclerosis. J Neuropathol Exp Neurol 66:819–827
Schoonheim MM, Hulst HE, Brandt RB, Strik M, Wink AM, Uitdehaag BM, Barkhof F, Geurts JJ (2015) Thalamus structure and function determine severity of cognitive impairment in multiple sclerosis. Neurology 84:776–783
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, Havrdova E, Hutchinson M, Kappos L, Lublin FD, Montalban X, O’Connor P, Sandberg-Wollheim M, Thompson AJ, Waubant E, Weinshenker B, Wolinsky JS (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69:292–302
Lechner-Scott J, Kappos L, Hofman M, Polman CH, Ronner H, Montalban X, Tintore M, Frontoni M, Buttinelli C, Amato MP, Bartolozzi ML, Versavel M, Dahlke F, Kapp JF, Gibberd R (2003) Can the expanded disability status scale be assessed by telephone? Mult Scler 9:154–159
Hulst HE, Schoonheim MM, Roosendaal SD, Popescu V, Schweren LJ, van der Werf YD, Visser LH, Polman CH, Barkhof F, Geurts JJ (2012) Functional adaptive changes within the hippocampal memory system of patients with multiple sclerosis. Hum Brain Mapp 33:2268–2280
Soldatos CR, Dikeos DG, Paparrigopoulos TJ (2000) Athens Insomnia Scale: validation of an instrument based on ICD-10 criteria. J Psychosom Res 48:555–560
Mulder JL, Dekker PH, Dekker R (1996) Verbale Leer-en Geheugentest. Swets and Zeitlinger, Lisse
Jolles J, Houx PJ, van Boxtel MPJ, Ponds RWHM (1995) Maastricht aging study: determinants of cognitive aging. Neuropsychological Publishers, Maastricht
Bucks R, Willison J (1997) Development and validation of the location learning test (LLT): a test of visuo-spatial learning designed for use with older adults and in dementia. Clin Neuropsychol 11:273–286
Wechsler D (1997) Wechsler Adult Intelligence Scale—administration and scoring manual. The Psychological Corporation, San Antonio
Lezak MD, Howieson DB, Loring DW (2004) Neuropsychological assessment. Oxford University Press, New York
Zigmond AS, Snaith RP (1983) The hospital anxiety and depression scale. Acta Psychiatr Scand 67:361–370
Vercoulen JH, Swanink CM, Fennis JF, Galama JM, van der Meer JW, Bleijenberg G (1994) Dimensional assessment of chronic fatigue syndrome. J Psychosom Res 38:383–392
Stewart AL, Greenfield S, Hays RD, Wells K, Rogers WH, Berry SD, McGlynn EA, Ware JE Jr (1989) Functional status and well-being of patients with chronic conditions. Results from the medical outcomes study. JAMA 262:907–913
Smith SM, Zhang Y, Jenkinson M, Chen J, Matthews PM, Federico A, De SN (2002) Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage 17:479–489
Patenaude B, Smith SM, Kennedy DN, Jenkinson M (2011) A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage 56:907–922
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15:273–289
Stam CJ, van Dijk BW (2002) Synchronization likelihood: an unbiased measure of generalized synchronization in multivariate data sets. Phys D 163:236–251
Kronholm E, Sallinen M, Suutama T, Sulkava R, Era P, Partonen T (2009) Self-reported sleep duration and cognitive functioning in the general population. J Sleep Res 18:436–446
Hulst HE, Gehring K, Uitdehaag BM, Visser LH, Polman CH, Barkhof F, Sitskoorn MM, Geurts JJ (2014) Indicators for cognitive performance and subjective cognitive complaints in multiple sclerosis: a role for advanced MRI? Mult Scler 20:1131–1134
De Havas JA, Parimal S, Soon CS, Chee MW (2012) Sleep deprivation reduces default mode network connectivity and anti-correlation during rest and task performance. Neuroimage 59:1745–1751
Killgore WD, Vanuk JR, Knight SA, Markowski SM, Pisner D, Shane B, Fridman A, Alkozei A (2015) Daytime sleepiness is associated with altered resting thalamocortical connectivity. NeuroReport 26:779–784
Thomas M, Sing H, Belenky G, Holcomb H, Mayberg H, Dannals R, Wagner H, Thorne D, Popp K, Rowland L, Welsh A, Balwinski S, Redmond D (2000) Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res 9:335–352
Most EI, Aboudan S, Scheltens P, Van Someren EJ (2012) Discrepancy between subjective and objective sleep disturbances in early- and moderate-stage Alzheimer disease. Am J Geriatr Psychiatry 20:460–467
Braley TJ, Kratz AL, Kaplish N, Chervin RD (2016) Sleep and cognitive function in multiple sclerosis. Sleep 39:1525–1533
Acknowledgments
This research has been executed within the VUmc MS Center Amsterdam and was sponsored by the Dutch MS Research Foundation, Grant Numbers 02-358b, 05-358c, 08-648, and 08-650.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Mr. van Geest serves as a consultant for Novartis.
Ms. Westerik reports no disclosures.
Dr. van der Werf received a speakers fee from Lundbeck in 2014 and performs contract research sponsored by Photopharmics, Inc., USA.
Dr. Geurts serves on the editorial boards of MS Journal, BMC Neurology, MS International and Neurology and the Scientific Advisory Board of the Dutch MS Research Foundation and of MS Academia, Merck-Serono and has served as a consultant for Merck-Serono, Biogen Idec, Novartis, Genzyme and Teva Pharmaceuticals.
Dr. Hulst receives research support from the Dutch MS Research Foundation, Grant Number 08-648 and serves as a consultant for Genzyme, Merck-Serono, Teva Pharmaceuticals and Novartis.
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Q. van Geest and B. Westerik contributed equally to this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
van Geest, Q., Westerik, B., van der Werf, Y.D. et al. The role of sleep on cognition and functional connectivity in patients with multiple sclerosis. J Neurol 264, 72–80 (2017). https://doi.org/10.1007/s00415-016-8318-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-016-8318-6