Abstract
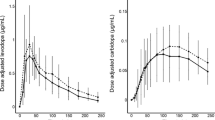
The differential diagnosis between multiple system atrophy with predominant parkinsonism (MSA-P) and Parkinson’s disease (PD) may be challenging at disease onset. Levodopa responsiveness helps distinguish the two groups, but studies evaluating this issue using objective standardized tests are scanty. We retrospectively examined the extent of levodopa response by an objective kinetic-dynamic test in a series of patients prospectively followed up for a parkinsonian syndrome and eventually diagnosed as MSA-P or PD. Sixteen MSA-P and 31 PD patients under chronic levodopa therapy received a first morning fasting dose of levodopa/benserazide (100/25 mg) or levodopa/carbidopa (125/12.5 or 100/25 mg) and underwent simultaneous serial assessments of plasma levodopa concentration and alternate finger tapping frequency up to 3 h post dosing. The main levodopa pharmacodynamic variables were the maximum percentage increase in tapping frequency over baseline values (ΔTapmax %) and the area under the tapping effect–time curve (AUCTap). Levodopa pharmacokinetics did not show significant differences between MSA-P and PD, whereas both the magnitude and overall extent of levodopa tapping effect were markedly reduced in the MSA-P group (p < 0.001). The combined use of specific cut-off values for both the main pharmacodynamic variables, ΔTapmax % <20 % and AUCTap <1900 [(tapping/min)·min], correctly discriminated 15 out of 16 MSA-P patients from PD patients. A combined estimation of these pharmacodynamic variables after a subacute low levodopa dose may be a simple and practical clinical tool to aid the differential diagnosis between MSA-P and PD.

Similar content being viewed by others
References
Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, Wood NW, Colosimo C, Dürr A, Fowler CJ, Kaufmann H, Klockgether T, Lees A, Poewe W, Quinn N, Revesz T, Robertson D, Sandroni P, Seppi K, Vidailhet M (2008) Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71:670–676
Fanciulli A, Wenning GK (2015) Multiple-system atrophy. N Engl J Med 372:249–263
Baschieri F, Calandra-Buonaura G, Doria A, Mastrolilli F, Palareti A, Barletta G, Solieri L, Guaraldi P, Martinelli P, Cortelli P (2015) Cardiovascular autonomic testing performed with a new integrated instrumental approach is useful in differentiating MSA-P from PD at an early stage. Parkinsonism Relat Disord 21:477–482
Gibb WR, Lees AJ (1988) The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry 51:745–752
Gelb DJ, Oliver E, Gilman S (1999) Diagnostic criteria for Parkinson disease. Arch Neurol 56:33–39
Wenning GK, Tison F, Ben Shlomo Y, Daniel SE, Quinn NP (1997) Multiple system atrophy: a review of 203 pathologically proven cases. Mov Disord 12:133–147
Gilman S, May SJ, Shults CW, Tanner CM, Kukull W, Lee VM, Masliah E, Low P, Sandroni P, Trojanowski JQ, Ozelius L, Foroud T (2005) North American Multiple System Atrophy Study Group. J Neural Transm 112:1687–1694
Köllensperger M, Geser F, Ndayisaba JP, Boesch S, Seppi K, Ostergaard K, Dupont E, Cardozo A, Tolosa E, Abele M, Klockgether T, Yekhlef F, Tison F, Daniels C, Deuschl G, Coelho M, Sampaio C, Bozi M, Quinn N, Schrag A, Mathias CJ, Fowler C, Nilsson CF, Widner H, Schimke N, Oertel W, Del Sorbo F, Albanese A, Pellecchia MT, Barone P, Djaldetti R, Colosimo C, Meco G, Gonzalez-Mandly A, Berciano J, Gurevich T, Giladi N, Galitzky M, Rascol O, Kamm C, Gasser T, Siebert U, Poewe W, Wenning GK, EMSA-SG (2010) Presentation, diagnosis, and management of multiple system atrophy in Europe: final analysis of the European multiple system atrophy registry. Mov Disord 25:2604–2612
Wenning GK, Geser F, Krismer F, Seppi K, Duerr S, Boesch S, Köllensperger M, Goebel G, Pfeiffer KP, Barone P, Pellecchia MT, Quinn NP, Koukouni V, Fowler CJ, Schrag A, Mathias CJ, Giladi N, Gurevich T, Dupont E, Ostergaard K, Nilsson CF, Widner H, Oertel W, Eggert KM, Albanese A, del Sorbo F, Tolosa E, Cardozo A, Deuschl G, Hellriegel H, Klockgether T, Dodel R, Sampaio C, Coelho M, Djaldetti R, Melamed E, Gasser T, Kamm C, Meco G, Colosimo C, Rascol O, Meissner WG, Tison F, Poewe W, European Multiple System Atrophy Study Group (2013) The natural history of multiple system atrophy: a prospective European cohort study. Lancet Neurol 12:264–274
Hughes AJ, Colosimo C, Kleedorfer B, Daniel SE, Lees AJ (1992) The dopaminergic response in multiple system atrophy. J Neurol Neurosurg Psychiatry 55:1009–1013
Parati EA, Fetoni V, Geminiani GC, Soliveri P, Giovannini P, Testa D, Genitrini S, Caraceni T, Girotti F (1993) Response to L-DOPA in multiple system atrophy. Clin Neuropharmacol 16:139–144
Contin M, Riva R, Martinelli P, Albani F, Avoni P, Baruzzi A (2001) Levodopa therapy monitoring in patients with Parkinson disease: a kinetic-dynamic approach. Ther Drug Monit 23:621–629
Contin M, Riva R, Martinelli P, Cortelli P, Albani F, Baruzzi A (1998) A levodopa kinetic-dynamic study of the rate of progression in Parkinson’s disease. Neurology 51:1075–1080
Contin M, Martinelli P, Riva R, Dondi M, Fanti S, Pettinato C, Scaglione C, Albani F, Baruzzi A (2003) Assessing dopaminergic function in Parkinson’s disease: levodopa kinetic-dynamic modeling and SPECT. J Neurol 250:1475–1481
Fahn S, Oakes D, Shoulson I, Kieburtz K, Rudolph A, Lang A, Olanow CW, Tanner C, Marek K (2004) Levodopa and the progression of Parkinson’s disease. N Engl J Med 351:2498–2508
Baruzzi A, Contin M, Albani F, Riva R (1986) Simple and rapid micromethod for the determination of levodopa and 3-O-methyldopa in human plasma by high-performance liquid chromatography with coulometric detection. J Chromatogr 375:165–169
Hagell P, Widner H (1999) Clinical rating of dyskinesias in Parkinson’s disease: use and reliability of a new rating scale. Mov Disord 14:448–455
Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, Cheshire WP, Chelimsky T, Cortelli P, Gibbons CH, Goldstein DS, Hainsworth R, Hilz MJ, Jacob G, Kaufmann H, Jordan J, Lipsitz LA, Levine BD, Low PA, Mathias C, Raj SR, Robertson D, Sandroni P, Schatz I, Schondorff R, Stewart JM, van Dijk JG (2011) Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res 21:69–72
Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE (2010) Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord 25:2649–2653
Nutt JG, Woodward WR, Carter JH, Gancher ST (1992) Effect of long-term therapy on the pharmacodynamics of levodopa. Relation to on-off phenomenon. Arch Neurol 49:1123–1130
Colosimo C, Merello M, Hughes AJ, Sieradzan K, Lees AJ (1996) Motor response to acute dopaminergic challenge with apomorphine and levodopa in Parkinson’s disease: implications for the pathogenesis of the on-off phenomenon. J Neurol Neurosurg Psychiatry 60:634–637
Harder S, Baas H (1998) Concentration-response relationship of levodopa in patients at different stages of Parkinson’s disease. Clin Pharmacol Ther 64:183–191
Fearnley JM, Lees AJ (1990) Striatonigral degeneration. A clinicopathological study. Brain 113:1823–1842
Ramaker C, Marinus J, Stiggelbout AM, van Hilten BJ (2002) Systematic evaluation of rating scales for impairment and disability in Parkinson’s disease. Mov Disord 17:867–876
Robertson DR, Renwick AG, Macklin B, Jones S, Waller DG, George CF, Fleming JS (1992) The influence of levodopa on gastric emptying in healthy eldery volunteers. Eur J Clin Pharmacol 42:409–412
Defer GL, Widner H, Marie RM, Remy P, Levivier M (1999) Core assessment program for surgical interventional therapies in Parkinson’s disease (CAPSIT-PD). Mov Disord 14:572–584
Michely J, Volz LJ, Barbe MT, Hoffstaedter F, Viswanathan S, Timmermann L, Eickhoff SB, Fink GR, Grefkes C (2005) Dopaminergic modulation of motor network dynamics in Parkinson’s disease. Brain 138:664–678
Agostino R, Berardelli A, Currà A, Accornero N, Manfredi M (1998) Clinical impairment of sequential finger movements in Parkinson’s disease. Mov Disord 13:418–421
Pal PK, Lee CS, Samii A, Schulzer M, Stoessl AJ, Mak EK, Wudel J, Dobko T, Tsui JK (2001) Alternating two finger tapping with contralateral activation is an objective measure of clinical severity in Parkinson’s disease and correlates with PET. Parkinsonism Relat Disord 7:305–309
Potter-Nerger M, Wenzelburger R, Deuschl G, Volkmann J (2009) Impact of subthalamic stimulation and medication on proximal and distal bradykinesia in Parkinson’s disease. Eur Neurol 62:114–119
Homann CN, Suppan K, Wenzel K, Giovannoni G, Ivanic G, Horner S, Ott E, Hartung HP (2000) The Bradykinesia Akinesia Incoordination Test (BRAIN TEST), an objective and user-friendly means to evaluate patients with parkinsonism. Mov Disord 15:641–647
Taylor Tavares AL, Jefferis GS, Koop M, Hill BC, Hastie T, Heit G, Bronte-Stewart HM (2005) Quantitative measurements of alternating finger tapping in Parkinson’s disease correlate with UPDRS motor disability and reveal the improvement in fine motor control from medication and deep brain stimulation. Mov Disord 20:1286–1298
Sambati L, Calandra-Buonaura G, Doria A, Cortelli P (2015) Diagnosis and management of autonomic failure in neurodegenerative disorders. Eur Neurol 73:126–133
Acknowledgments
The skilful nursing and technical assistance of Anita Calisti and Carmina Candela are gratefully acknowledged. Anne Prudence Collins edited the English text. No specific funding was received to perform this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical standards
The study was approved by the DIBINEM—University of Bologna institutional review board and has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Patients had given their written informed consent to personal data processing for research purposes.
Rights and permissions
About this article
Cite this article
Calandra-Buonaura, G., Doria, A., Lopane, G. et al. Pharmacodynamics of a low subacute levodopa dose helps distinguish between multiple system atrophy with predominant Parkinsonism and Parkinson’s disease. J Neurol 263, 250–256 (2016). https://doi.org/10.1007/s00415-015-7961-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-015-7961-7