Abstract
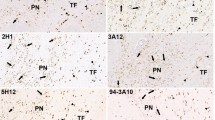
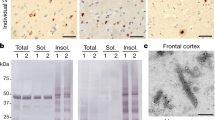
Pathological changes in corticobasal degeneration (CBD) consist of abnormal deposition of the microtubule-associated protein tau. However, the simultaneous accumulation of different misfolded proteins in the brain can be observed in many neurodegenerative diseases with significantly longer disease durations. We encountered a patient with CBD who survived for an extremely long period (18 years) after the diagnosis. We performed an autopsy to elucidate the effect of the longer survival on the pathology of CBD. We observed abnormal aggregation of trans-activating response region DNA-binding protein of 43 kDa (TDP-43) and α-synuclein, as well as phosphorylated tau, in neurons of broader regions of the brain, beyond the amygdala and other limbic areas. We found that phosphorylated tau, α-synuclein, and TDP-43 partially co-existed in the same cellular aggregates. The triple pathologic changes might be related to the longer survival of the patient compared with the typical clinical course of patients with CBD. Further investigations are required to support the hypothesis that tauopathy, synucleinopathy, and TDP-43 proteinopathy might share common pathogenic mechanisms in terms of cross-seeding of the pathologic proteins.




Similar content being viewed by others
References
Rebeiz JJ, Kolodny EH, Richardson EP Jr (1968) Corticodentatonigral degeneration with neuronal achromasia. Arch Neurol 18:20–33
Boeve BF, Lang AE, Litvan I (2003) Corticobasal degeneration and its relationship to progressive supranuclear palsy and frontotemporal dementia. Ann Neurol 54(Suppl 5):S15–S19
Dickson DW, Bergeron C, Chin SS et al (2002) Office of Rare Diseases neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol 61:935–946
Feany MB, Dickson DW (1995) Widespread cytoskeletal pathology characterizes corticobasal degeneration. Am J Pathol 146:1388–1396
Kouri N, Murray ME, Hassan A et al (2011) Neuropathological features of corticobasal degeneration presenting as corticobasal syndrome or Richardson syndrome. Brain 134:3264–3275
Ling H, O’Sullivan SS, Holton JL et al (2010) Does corticobasal degeneration exist? A clinicopathological re-evaluation. Brain 133:2045–2057
Aiba I (2012) Corticobasal syndrome: recent advances and future directions. Brain Nerve 64:462–473
Wenning GK, Litvan I, Jankovic J et al (1998) Natural history and survival of 14 patients with corticobasal degeneration confirmed at postmortem examination. J Neurol Neurosurg Psychiatry 64:184–189
Uryu K, Nakashima-Yasuda H, Forman MS et al (2008) Concomitant TAR-DNA-binding protein 43 pathology is present in Alzheimer disease and corticobasal degeneration but not in other tauopathies. J Neuropathol Exp Neurol 67:555–564
Arai T, Ikeda K, Akiyama H et al (2004) Identification of amino-terminally cleaved tau fragments that distinguish progressive supranuclear palsy from corticobasal degeneration. Ann Neurol 55:72–79
Tsuji H, Arai T, Kametani F et al (2012) Molecular analysis and biochemical classification of TDP-43 proteinopathy. Brain 135:3380–3391
Mackenzie IR, Neumann M, Baborie A et al (2011) A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol 122:111–113
Braak H, Del Tredici K, Rub U et al (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211
Galpern WR, Lang AE (2006) Interface between tauopathies and synucleinopathies: a tale of two proteins. Ann Neurol 59:449–458
Clarimon J, Molina-Porcel L, Gomez-Isla T et al (2009) Early-onset familial Lewy body dementia with extensive tauopathy: a clinical, genetic, and neuropathological study. J Neuropathol Exp Neurol 68:73–82
Duda JE, Giasson BI, Mabon ME et al (2002) Concurrence of alpha-synuclein and tau brain pathology in the Contursi kindred. Acta Neuropathol 104:7–11
Galloway PG, Grundke-Iqbal I, Iqbal K, Perry G (1988) Lewy bodies contain epitopes both shared and distinct from Alzheimer neurofibrillary tangles. J Neuropathol Exp Neurol 47:654–663
Giasson BI, Forman MS, Higuchi M et al (2003) Initiation and synergistic fibrillization of tau and alpha-synuclein. Science 300:636–640
Ishizawa T, Mattila P, Davies P, Wang D, Dickson DW (2003) Colocalization of tau and alpha-synuclein epitopes in Lewy bodies. J Neuropathol Exp Neurol 62:389–397
Terni B, Rey MJ, Boluda S et al (2007) Mutant ubiquitin and p62 immunoreactivity in cases of combined multiple system atrophy and Alzheimer’s disease. Acta Neuropathol 113:403–416
Wills J, Jones J, Haggerty T et al (2010) Elevated tauopathy and alpha-synuclein pathology in postmortem Parkinson’s disease brains with and without dementia. Exp Neurol 225:210–218
Badiola N, de Oliveira RM, Herrera F et al (2011) Tau enhances alpha-synuclein aggregation and toxicity in cellular models of synucleinopathy. PLoS One 6:e26609
Iseki E, Marui W, Kosaka K, Ueda K (1999) Frequent coexistence of Lewy bodies and neurofibrillary tangles in the same neurons of patients with diffuse Lewy body disease. Neurosci Lett 265:9–12
Hamilton RL (2000) Lewy bodies in Alzheimer’s disease: a neuropathological review of 145 cases using alpha-synuclein immunohistochemistry. Brain Pathol 10:378–384
Lippa CF, Fujiwara H, Mann DM et al (1998) Lewy bodies contain altered alpha-synuclein in brains of many familial Alzheimer’s disease patients with mutations in presenilin and amyloid precursor protein genes. Am J Pathol 153:1365–1370
Raghavan R, Khin-Nu C, Brown A et al (1993) Detection of Lewy bodies in Trisomy 21 (Down’s syndrome). Can J Neurol Sci 20:48–51
Lippa CF, Schmidt ML, Lee VM, Trojanowski JQ (1999) Antibodies to alpha-synuclein detect Lewy bodies in many down’s syndrome brains with Alzheimer’s disease. Ann Neurol 45:353–357
Judkins AR, Forman MS, Uryu K et al (2002) Co-occurrence of Parkinson’s disease with progressive supranuclear palsy. Acta Neuropathol 103:526–530
Forman MS, Schmidt ML, Kasturi S et al (2002) Tau and alpha-synuclein pathology in amygdala of Parkinsonism-dementia complex patients of Guam. Am J Pathol 160:1725–1731
Wilhelmsen KC, Forman MS, Rosen HJ et al (2004) 17q-linked frontotemporal dementia-amyotrophic lateral sclerosis without tau mutations with tau and alpha-synuclein inclusions. Arch Neurol 61:398–406
Yancopoulou D, Xuereb JH, Crowther RA, Hodges JR, Spillantini MG (2005) Tau and alpha-synuclein inclusions in a case of familial frontotemporal dementia and progressive aphasia. J Neuropathol Exp Neurol 64:245–253
Schmidt ML, Martin JA, Lee VM, Trojanowski JQ (1996) Convergence of Lewy bodies and neurofibrillary tangles in amygdala neurons of Alzheimer’s disease and Lewy body disorders. Acta Neuropathol 91:475–481
Mooney T, Tampiyappa A, Robertson T et al (2011) Dual pathology of corticobasal degeneration and Parkinson’s disease in a patient with clinical features of progressive supranuclear palsy. Neurol India 59:887–890
Kouri N, Oshima K, Takahashi M et al (2013) Corticobasal degeneration with olivopontocerebellar atrophy and TDP-43 pathology: an unusual clinicopathologic variant of CBD. Acta Neuropathol 125:741–752
Jellinger KA (2011) Interaction between alpha-synuclein and other proteins in neurodegenerative disorders. Sci World J 11:1893–1907
Jellinger KA (2012) Interaction between pathogenic proteins in neurodegenerative disorders. J Cell Mol Med 16:1166–1183
Acknowledgments
We would like to thank Ms. Mika Oka and the members of Department of Neurology, Kumamoto University Hospital for technical help with autopsy analyses and clinical data collection. This work was supported by a Grant-in-Aid for Scientific Research, the Ministry of Education, Culture, Sports, Science and Technology of Japan, and Grants-in-Aid from the Amyloidosis Research Committee, the Ministry of Health, Labour and Welfare of Japan.
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical standard
This study has been approved by the appropriate ethics committee and has therefore been performed in the accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. The spouse of the patient gave informed consent instead of the patient.
Author information
Authors and Affiliations
Corresponding author
Additional information
S. Yamashita, N. Sakashita and T. Yamashita authors contributed equally to the manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yamashita, S., Sakashita, N., Yamashita, T. et al. Concomitant accumulation of α-synuclein and TDP-43 in a patient with corticobasal degeneration. J Neurol 261, 2209–2217 (2014). https://doi.org/10.1007/s00415-014-7491-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-014-7491-8