Abstract
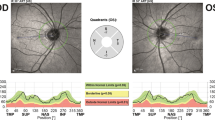
To define the retinal phenotype of subjects with familial dysautonomia (FD). A cross-sectional study was carried out in 90 subjects divided in three groups of 30 each (FD subjects, asymptomatic carriers and controls). The study was developed at the Dysautonomia Center, New York University Medical Center. All subjects underwent spectral domain optical coherence tomography (OCT) and full neuro-ophthalmic examinations. In a subset of affected subjects, visual evoked potentials and microperimetry were also obtained. We compared the retinal nerve fiber layer (RNFL) thickness from OCT between the three groups. OCT showed loss of the RNFL in all FD subjects predominantly in the maculopapillary region (63 % temporally, p < 0.0001; and 21 % nasally, p < 0.005). RNFL loss was greatest in older FD subjects and was associated with decreased visual acuity and color vision, central visual field defects, temporal optic nerve pallor, and delayed visual evoked potentials. Asymptomatic carriers of the FD gene mutation all had thinner RNFL (12 % globally, p < 0.005). OCT and clinical neuro-ophthalmological findings suggest that maculopapillary ganglion cells are primarily affected in FD subjects, leading to a specific optic nerve damage that closely resembles mitochondrial optic neuropathies. This raises the possibility that reduced IKAP levels may affect mitochondrial proteins and their function in the nervous system, particularly in the retina.





Similar content being viewed by others
References
Riley CM, Day RA, Greeley DM, Landford WS (1949) Central autonomic dysfunction with defective lacrimation: I. Report of five cases. Pediatrics 3(4):468–478
Dong J, Edelmann L, Bajwa AM et al (2002) Familial dysautonomia: detection of the IKBKAP IVS20(+6T–>C) and R696P mutations and frequencies among Ashkenazi Jews. Am J Med Genet 110(3):253–257
Mezey E, Parmalee A, Szalayova I et al (2003) Of splice and men: what does the distribution of IKAP mRNA in the rat tell us about the pathogenesis of familial dysautonomia? Brain Res 983(1–2):209–214
Anderson SL, Coli R, Daly IW et al (2001) Familial dysautonomia is caused by mutations of the IKAP gene. Am J Hum Genet 68(3):753–758
Slaugenhaupt SA, Blumenfeld A, Gill SP et al (2001) Tissue-specific expression of a splicing mutation in the IKBKAP gene causes familial dysautonomia. Am J Hum Genet 68(3):598–605
Smith AA, Dancis J (1964) Taste discrimination in familial dysautonomia. Pediatrics 33:441–443
Norcliffe-Kaufmann L, Axelrod F, Kaufmann H (2010) Afferent baroreflex failure in familial dysautonomia. Neurology 75(21):1904–1911
Axelrod FB, Iyer K, Fish I et al (1981) Progressive sensory loss in familial dysautonomia. Pediatrics 67(4):517–522
Norcliffe-Kaufmann L, Kaufmann H (2012) Familial dysautonomia (Riley–Day syndrome): when baroreceptor feedback fails. Auton Neurosci 172(1–2):26–30
Macefield VG, Norcliffe-Kaufmann L, Axelrod FB, Kaufmann H (2013) Cardiac-locked bursts of muscle sympathetic nerve activity are absent in familial dysautonomia. J Physiol 591(Pt 3):689–700
Macefield VG, Norcliffe-Kaufmann L, Gutierrez J et al (2011) Can loss of muscle spindle afferents explain the ataxic gait in Riley–Day syndrome? Brain 134(Pt 11):3198–3208
Liebman SD (1956) Ocular manifestations of Riley–Day syndrome; familial autonomic dysfunction. AMA Arch Ophthalmol 56(5):719–725
Kroop IG (1956) The production of tears in familial dysautonomia; preliminary report. J Pediatr 48(3):328–329
Josaitis CA, Matisoff M (2002) Familial dysautonomia in review: diagnosis and treatment of ocular manifestations. Adv Exp Med Biol 506(Pt A):71–80
Liebman SD (1968) Riley–Day syndrome: long-term ophthalmologic observations. Trans Am Ophthalmol Soc 66:95–116
Goldberg MF, Payne JW, Brunt PW (1968) Ophthalmologic studies of familial dysautonomia. The Riley–Day syndrome. Arch Ophthalmol 80(6):732–743
Mendoza-Santiesteban CE, Hedges TR 3rd, Norcliffe-Kaufmann L et al (2012) Clinical neuro-ophthalmic findings in familial dysautonomia. J Neuroophthalmol 32(1):23–26
Verdina T, Giacomelli G, Sodi A, et al. Biofeedback rehabilitation of eccentric fixation in patients with Stargardt disease. Eur J Ophthalmol 2013:0
Tarita-Nistor L, Gonzalez EG, Markowitz SN, Steinbach MJ (2009) Plasticity of fixation in patients with central vision loss. Vis Neurosci 26(5–6):487–494
Rizzo JF 3rd, Lessell S, Liebman SD (1986) Optic atrophy in familial dysautonomia. Am J Ophthalmol 102(4):463–467
Diamond GA, D’Amico RA, Axelrod FB (1987) Optic nerve dysfunction in familial dysautonomia. Am J Ophthalmol 104(6):645–648
Groom M, Kay MD, Corrent GF (1997) Optic neuropathy in familial dysautonomia. J Neuroophthalmol 17(2):101–102
Fraser JA, Biousse V, Newman NJ (2010) The neuro-ophthalmology of mitochondrial disease. Surv Ophthalmol 55(4):299–334
Kesler A, Pianka P (2003) Toxic optic neuropathy. Curr Neurol Neurosci Rep 3(5):410–414
Yu-Wai-Man P, Griffiths PG, Chinnery PF (2011) Mitochondrial optic neuropathies—disease mechanisms and therapeutic strategies. Prog Retin Eye Res 30(2):81–114
Wang MY, Sadun AA (2013) Drug-related mitochondrial optic neuropathies. J Neuroophthalmol 33(2):172–178
Sadun AA, Win PH, Ross-Cisneros FN et al (2000) Leber’s hereditary optic neuropathy differentially affects smaller axons in the optic nerve. Trans Am Ophthalmol Soc 98:223–232 (discussion 32–5)
Sadun AA, Martone JF, Muci-Mendoza R et al (1994) Epidemic optic neuropathy in Cuba. Eye findings. Arch Ophthalmol 112(5):691–699
Amati-Bonneau P, Milea D, Bonneau D et al (2009) OPA1-associated disorders: phenotypes and pathophysiology. Int J Biochem Cell Biol 41(10):1855–1865
Hedges TR 3rd, Hirano M, Tucker K, Caballero B (1997) Epidemic optic and peripheral neuropathy in Cuba: a unique geopolitical public health problem. Surv Ophthalmol 41(4):341–353
Yu-Wai-Man P, Bailie M, Atawan A et al (2011) Pattern of retinal ganglion cell loss in dominant optic atrophy due to OPA1 mutations. Eye (Lond) 25(5):596–602
Barboni P, Savini G, Parisi V et al (2011) Retinal nerve fiber layer thickness in dominant optic atrophy measurements by optical coherence tomography and correlation with age. Ophthalmology 118(10):2076–2080
Barboni P, Savini G, Valentino ML et al (2005) Retinal nerve fiber layer evaluation by optical coherence tomography in Leber’s hereditary optic neuropathy. Ophthalmology 112(1):120–126
Newman NJ (2005) Hereditary optic neuropathies: from the mitochondria to the optic nerve. Am J Ophthalmol 140(3):517–523
Sadun AA, Carelli V, Salomao SR et al (2003) Extensive investigation of a large Brazilian pedigree of 11778/haplogroup J Leber hereditary optic neuropathy. Am J Ophthalmol 136(2):231–238
Cohn AC, Toomes C, Hewitt AW et al (2008) The natural history of OPA1-related autosomal dominant optic atrophy. Br J Ophthalmol 92(10):1333–1336
Rubin BY, Anderson SL (2008) The molecular basis of familial dysautonomia: overview, new discoveries and implications for directed therapies. Neuromol Med 10(3):148–156
Lee G, Papapetrou EP, Kim H et al (2009) Modelling pathogenesis and treatment of familial dysautonomia using patient-specific iPSCs. Nature 461(7262):402–406
Axelrod FB, Liebes L, Gold-Von Simson G et al (2011) Kinetin improves IKBKAP mRNA splicing in patients with familial dysautonomia. Pediatr Res 70(5):480–483
Barboni P, Savini G, Feuer WJ et al (2012) Retinal nerve fiber layer thickness variability in Leber hereditary optic neuropathy carriers. Eur J Ophthalmol 22(6):985–991
Acknowledgments
We would like to thank all the patients and families who participated in this study. This work was supported by the Dysautonomia Foundation Inc., the National Institutes of Health (U54-NS065736-01) and The Massachusetts Lions Clubs/Research to Prevent Blindness Challenge Grant.
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mendoza-Santiesteban, C.E., Hedges III, T.R., Norcliffe-Kaufmann, L. et al. Selective retinal ganglion cell loss in familial dysautonomia. J Neurol 261, 702–709 (2014). https://doi.org/10.1007/s00415-014-7258-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-014-7258-2