Abstract
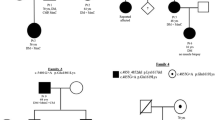
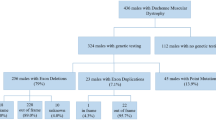
Since the initial description in 2010 of anoctamin 5 deficiency as a cause of muscular dystrophy, a handful of papers have described this disease in cases of mixed populations. We report the first large regional study and present data on new aspects of prevalence, muscular and cardiac phenotypic characteristics, and muscle protein expression. All patients in our neuromuscular unit with genetically unclassified, recessive limb girdle muscular dystrophy (LGMD2), Miyoshi-type distal myopathy (MMD) or persistent asymptomatic hyperCK-emia (PACK) were assessed for mutations in the ANO5 gene. Genetically confirmed patients were evaluated with muscular and cardiopulmonary examination. Among 40 unclassified patients (28 LGMD2, 5 MMD, 7 PACK), 20 were homozygous or compound heterozygous for ANO5 mutations, (13 LGMD2, 5 MMD, 2 PACK). Prevalence of ANO5 deficiency in Denmark was estimated at 1:100.000 and ANO5 mutations caused 11 % of our total cohort of LGMD2 cases making it the second most common LGMD2 etiology in Denmark. Eight patients complained of dysphagia and 3 dated symptoms of onset in childhood. Cardiac examinations revealed increased frequency of premature ventricular contractions. Four novel putative pathogenic mutations were discovered. Total prevalence and distribution of phenotypes of ANO5 disease in a representative regional cohort are described for the first time. A high prevalence of ANO5 deficiency was found among patients with unclassified LGMD2 (46 %) and MMD (100 %). The high incidence of reported dysphagia is a new phenotypic feature not previously reported, and cardiac investigations revealed that ANO5-patients may have an increased risk of ventricular arrhythmia.


Similar content being viewed by others
References
Anderson LV, Davison K (1999) Multiplex Western blotting system for the analysis of muscular dystrophy proteins. Am J Pathol 154:1017–1022
Behin A, Laforet P, Cossee M, Deburgrave N, Orhant L, Kaplan JC, Stojkovic T, leturcq F, Eynard B (2011) Myopathies with mutations in anoctamin 5: Phenotype and gene mutations in a French cohort. p 675
Bolduc V, Marlow G, Boycott KM, Saleki K, Inoue H, Kroon J, Itakura M, Robitaille Y, Parent L, Baas F, Mizuta K, Kamata N, Richard I, Linssen WH, Mahjneh I, de Visser M, Bashir R, Brais B (2010) Recessive mutations in the putative calcium-activated chloride channel Anoctamin 5 cause proximal LGMD2L and distal MMD3 muscular dystrophies. Am J Hum Genet 86:213–221
Duno M, Sveen ML, Schwartz M, Vissing J (2008) cDNA analyses of CAPN3 enhance mutation detection and reveal a low prevalence of LGMD2A patients in Denmark. Eur J Hum Genet 16:935–940
Hayashi YK, Engvall E, Rikawa-Hirasawa E, Goto K, Koga R, Nonaka I, Sugita H, Arahata K (1993) Abnormal localization of laminin subunits in muscular dystrophies. J Neurol Sci 119:53–64
Hicks D, Sarkozy A, Muelas N, Koehler K, Huebner A, Hudson G, Chinnery PF, Barresi R, Eagle M, Polvikoski T, Bailey G, Miller J, Radunovic A, Hughes PJ, Roberts R, Krause S, Walter MC, Laval SH, Straub V, Lochmuller H, Bushby K (2011) A founder mutation in Anoctamin 5 is a major cause of limb-girdle muscular dystrophy. Brain 134:171–182
Magri F, Bo RD, D’Angelo MG, Sciacco M, Gandossini S, Govoni A, Napoli L, Ciscato P, Fortunato F, Brighina E, Bonato S, Bordoni A, Lucchini V, Corti S, Moggio M, Bresolin N, Comi GP (2012) Frequency and characterisation of anoctamin 5 mutations in a cohort of Italian limb-girdle muscular dystrophy patients. Neuromuscul Disord 22:934–943
Penttila S, Palmio J, Suominen T, Raheem O, Evila A, Muelas GN, Tasca G, Waddell LB, Clarke NF, Barboi A, Hackman P, Udd B (2012) Eight new mutations and the expanding phenotype variability in muscular dystrophy caused by ANO5. Neurology 78:897–903
Sajadieh A, Nielsen OW, Rasmussen V, Hein HO, Frederiksen BS, Davanlou M, Hansen JF (2006) Ventricular arrhythmias and risk of death and acute myocardial infarction in apparently healthy subjects of age > or =55 years. Am J Cardiol 97:1351–1357
Sarkozy A, Deschauer M, Carlier RY, Schrank B, Seeger J, Walter MC, Schoser B, Reilich P, Leturq F, Radunovic A, Behin A, Laforet P, Eymard B, Schreiber H, Hicks D, Vaidya SS, Glaser D, Carlier PG, Bushby K, Lochmuller H, Straub V (2012) Muscle MRI findings in limb girdle muscular dystrophy type 2L. Neuromuscul Disord 22(Suppl 2):S122–S129
Schessl J, Kress W, Schoser B (2012) Novel ANO5 mutations causing hyper-CK-emia, limb girdle muscular weakness and Miyoshi type of muscular dystrophy. Muscle Nerve 45:740–742
Stubgen JP (2008) Facioscapulohumeral muscular dystrophy: a radiologic and manometric study of the pharynx and esophagus. Dysphagia 23:341–347
Sveen ML, Schwartz M, Vissing J (2006) High prevalence and phenotype-genotype correlations of limb girdle muscular dystrophy type 2I in Denmark. Ann Neurol 59:808–815
Tsutsumi S, Kamata N, Vokes TJ, Maruoka Y, Nakakuki K, Enomoto S, Omura K, Amagasa T, Nagayama M, Saito-Ohara F, Inazawa J, Moritani M, Yamaoka T, Inoue H, Itakura M (2004) The novel gene encoding a putative transmembrane protein is mutated in gnathodiaphyseal dysplasia (GDD). Am J Hum Genet 74:1255–1261
Udd B (2012) Distal myopathies–new genetic entities expand diagnostic challenge. Neuromuscul Disord 22:5–12
Wahbi K, Behin A, Becane HM, leturcq F, Cossee M, Laforet P, Stojkovic T, Carlier P, Toussaint M, Gaxotte V, Cluzel P, Eymard B, Duboc D (2012) Dilated cardiomyopathy in patients with mutations in anoctamin 5. Int J Cardiol [Epub ahead of print]
Witting N, Duno M, Vissing J (2013) Becker muscular dystrophy with widespread muscle hypertrophy and a non-sense mutation of exon 2. Neuromuscul Disord 23:–25
Conflicts of interest
Dr. Vissing has received research support, honoraria, and travel funding from the Genzyme Corporation, and is a member of the Global Advisory Board for Pompe Disease for Genzyme Corporation. Dr. Vissing has also worked as a consultant/advisor for Lundbeck Pharmaceutical Company, and as a drug safety consultant for Cardoz AB, Sweden.
Ethical standard
The patients were fully informed and agreed to participate and to publication of the results. The study was carried out according to the ethical standards laid down by the Helsinki declaration in 1964 and further on.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Witting, N., Duno, M., Petri, H. et al. Anoctamin 5 muscular dystrophy in Denmark: prevalence, genotypes, phenotypes, cardiac findings, and muscle protein expression. J Neurol 260, 2084–2093 (2013). https://doi.org/10.1007/s00415-013-6934-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-013-6934-y