Abstract
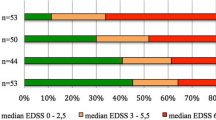
Longitudinally extensive transverse myelitis (LETM) is a syndrome with extensive spinal cord lesions spanning three or more vertebral segments on spinal cord MRI. Although many reports have indicated that LETM is a characteristic feature of neuoromyelitis optica (NMO) in Western countries, the clinical characteristics and risk for development of NMO in Korean patients with LETM is not clear. We retrospectively investigated the clinical, laboratory, radiological features, and prognosis of Korean patients with a first-ever episode of idiopathic LETM. Patients were classified into four subgroups, depending on their clinical course: monophasic LETM, recurrent LETM, NMO, and classic multiple sclerosis (MS). We compared various clinical, laboratory, and radiological features between groups. Of 20 patients with first-ever LETM, 15 (75%) were men, and 13 (65%) experienced clinical relapse over a mean follow-up period of 58 months. Three of 20 patients (two with NMO, one with recurrent myelitis) were seropositive for anti-AQP4 antibodies. The predominance of men in the monophasic and recurrent LETM groups compared to the NMO group was remarkable. In conclusion, Korean patients with LETM are predominantly male and have low seropositivity for anti-AQP4 antibody, which distinguishes them from LETM patients in Western countries. Patients with LETM and seropositivity for anti-AQP4 antibody have a high risk of relapse. The male predominance and the relatively low seropositive rate for anti-AQP4 suggests that rather than being a limited form of NMO, recurrent LETM is a new clinical entity in Koreans.

Similar content being viewed by others
References
Weinshenker B, Wingerchuk D, Vukusic S, Linbo L, Pittock SJ, Lucchinetti CF, Lennon VA (2006) Neuromyelitis optica IgG predicts relapse after longitudinally extensive transverse myelitis. Ann Neurol 59:566–569. doi:10.1002/ana.20770
Tartaglino LM, Friedman DP, Flanders AE, Lublin FD, Knobler RL, Liem M (1995) Multiple sclerosis in the spinal cord: MR appearance and correlation with clinical parameters. Radiology 195:725–732
Waters P, Jarius S, Littletone E, Leite MI, Jacob S, Gray B, Geraldes R, Vale T, Jacob A, Palace J, Maxwell S, Beeson D, Vincent A (2008) Aquaporin-4 antibodies in neuromyelitis optica and longitudinally extensive transverse myelitis. Arch Neurol 65:913–919
Chan KH, Ramsden DB, Yu YL, Kwok KH, Chu AC, Ho PW, Kwan JS, Lee R, Lim E, Kung MH, Ho SL (2009) Neuromyelitis optica-IgG in idiopathic inflammatory demyelinating disorders amongst Hong Kong, Chinese. Eur J Neurol 16:310–316. doi:10.1111/j.1468-1331.2008.02376
Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, Nakashima I, Weinshenekr BG (2004) A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 364:2106–2112. doi:10.1016/S0140-6736(04)17551-X
Pittock SJ, Lennon VA, de Seze J, Vermersch P, Homburger HA, Wingerchuk DM, Lucchinetti CF, Zephir H, Moder K, Weinshenker BG (2008) Neuromyelitis optica and non organ-specific autoimmunity. Arch Neurol 65:78–83
Matsuoka T, Matsushita T, Kawano Y, Osoegawa M, Ochi H, Ishizu T, Minohara M, Kikuchi H, Mihara F, Ohyagi Y, Kira J (2007) Heterogeneity of auaporin-4 autoimmunity and spinal cord lesions in multiple sclerosis in Japanese. Brain 130:1206–1223. doi:10.1093/brain/awm027
Kira J (2008) Neuromyelitis optica and Asian phenotype of multiple sclerosis. Ann NY Acad Sci 1142:58–71. doi:10.1196/annals.1444.002
Lee SS, Sohn EH, Nam SW (2006) Preliminary studies on the clinical features of multiple sclerosis in Korea. J Clin Neurol 2:231–237
Transverse Myelitis Consortium Working Group (2002) Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology 59:499–505
Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG (2006) Revised diagnostic criteria for neuromyelitis optica. Neurology 66:1485–1489. doi:10.1212/01.wnl.0000216139.44259.74
McDonald WI, Compston A, Edan G, Goodkin D, Hartnung H, Lublin FD, McFarland HF, Paty DW, Polman CH, Reingold SC, Sandberg-Wollheim M, Sibley W, Thompson A, van den Noort S, Weinshenker BY, Wolinsky JS (2001) Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol 50:121–127. doi:10.1002/ana.1032
Kim KK (2003) Idiopathic recurrent transverse myelitis. Arch Neurol 60:1290–1294
Krampla W, Aboul-Enein F, Jecel J, Lang W, Fertl E, Hruby W, Kristoferitsch W (2009) Spinal cord lesions in patients with neuromyelitis optica: a retrospective long-term MRI follow-up study. Eur Radiol 19:2535–2543. doi:10.1007/s00330-009-1425-1433
Scott TF, Bhagavatula K, Snyder PJ, Chieffe C (1998) Transverse myelitis. Comparison with spinal cord presentations of multiple sclerosis. Neurology 50:429–433
Lycklama G, Thompson A, Filippi M, Miller D, Polman C, Fazekas F, Barkhof F (2003) Spinal cord MRI in multiple sclerosis. Lancet Neurol 2:555–562. doi:10.1016/s1474-4422(03)00504-0
Losseff NA, Wang L, Miller DH, Thompson AJ (2001) T1 hypointensity of the spinal cord in multiple sclerosis. J Neurol 248:517–521. doi:10.1007/s004150170163
Su JJ, Osoegawa M, Matsuoka T, Mihohara M, Tanaka M, Ishizu T, Mihara F, Taniwaki T, Kira J (2006) Up-regulation of vascular growth factors in multiple sclerosis: correlation with MRI findings. J Neurol Sci 243:21–30. doi:10.1016/j.jns.2005.11.006
Qiu W, Wu JS, Zhang MN, Matsushita T, Kira JI, Carroll WM, Mastaglia Fl, Kermode AG (2009) Longitudinally extensive myelopathy in Caucasians: a west Australian study of 26 cases from the Perth demyelinating disease database. J Neurol Neurosurg Psychiatry 25 [Epub ahead of print]
Acknowledgments
This work was supported by Grant No. A091208 from the Korean Ministry for Health, Welfare, and Family Affairs funds.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, S.H., Kim, S.M., Vincent, A. et al. Clinical characteristics, prognosis, and seropositivity to the anti-aquaporin-4 antibody in Korean patients with longitudinally extensive transverse myelitis. J Neurol 257, 920–925 (2010). https://doi.org/10.1007/s00415-009-5438-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-009-5438-2