Abstract
Background
Spinal muscular atrophy (SMA) is caused by a homozygous deletion of the survival motor neuron (SMN)1 gene. The nearly identical SMN2 gene plays a disease modifying role. SMA is classified into four different subtypes based on age of onset and clinical course (SMA types 1–4). The natural history of early onset SMA types 1–3a has been studied extensively. Late onset SMA is rare and disease course has not been studied in detail.
Objective
To perform a prospective study on the clinical course and the correlation with SMN2 copy numbers of late onset SMA.
Methods
Patients fulfilling the diagnostic criteria for late onset SMA (types 3b and 4) were included in the study. At inclusion and follow-up, muscle strength, respiratory function, functional status and quality of life were assessed. SMN2 copy number was determined in all patients.
Results
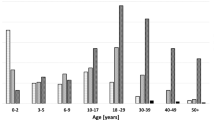
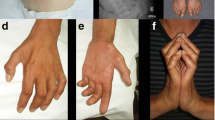
Twelve patients were identified and included. Six patients were siblings from one family, two patients were brothers from a second family and four patients were sporadic cases. All patients carried four copies of the SMN2 gene. Median age of disease onset was 22.2 years (10–37). Age of disease onset in patients from family one was lower as compared to the other patients. None of the outcome measures changed after a follow-up of 2.5 years. Five patients reported an increase in fatigue and muscle weakness. None of the patients showed symptoms of respiratory insufficiency.
Conclusions
This study indicates that late onset SMA is not characterized by disease progression and that alternative or surrogate disease markers are required for the design of future trials. This study confirms the finding that SMN2 copy number is a SMA disease course modifier.
Similar content being viewed by others
References
The Amyotrophic Lateral Sclerosis Functional Rating Scale. Assessment of activities of daily living in patients with amyotrophic lateral sclerosis. The ALS CNTF treatment study (ACTS) phase I–II Study Group. Arch Neurol 53:141–147
Brahe C, Vitali T, Tiziano FD, Angelozzi C, Pinto AM, Borgo F, Moscato U, Bertini E, Mercuri E, Neri G (2005) Phenylbutyrate increases SMN gene expression in spinal muscular atrophy patients. Eur J Hum Genet 13:256–259
Brichta L, Hofmann Y, Hahnen E, Siebzehnrubl FA, Raschke H, Blumcke I, Eyupoglu IY, Wirth B (2003) Valproic acid increases the SMN2 protein level: a well-known drug as a potential therapy for spinal muscular atrophy. Hum Mol Genet 12:2481–2489
Brichta L, Holker I, Haug K, Klockgether T, Wirth B (2006) In vivo activation of SMN in spinal muscular atrophy carriers and patients treated with valproate. Ann Neurol 59:970–975
Brooke MH, Griggs RC, Mendell JR, Fenichel GM, Shumate JB, Pellegrino RJ (1981) Clinical trial in Duchenne dystrophy. I. The design of the protocol. Muscle Nerve 4:186–197
Brooks D, Solway S, Gibbons WJ (2003) ATS statement on six-minute walk test. Am J Respir Crit Care Med 167:1287
Chung BH, Wong VC, Ip P (2004) Spinal muscular atrophy: survival pattern and functional status. Pediatrics 114:548–553
Escolar DM, Buyse G, Henricson E, Leshner R, Florence J, Mayhew J, Tesi- Rocha C, Gorni K, Pasquali L, Patel KM, McCarter R, Huang J, Mayhew T, Bertorini T, Carlo J, Connolly AM, Clemens PR, Goemans N, Iannaccone ST, Igarashi M, Nevo Y, Pestronk A, Subramony SH, Vedanarayanan VV, Wessel H (2005) CINRG randomized controlled trial of creatine and glutamine in Duchenne muscular dystrophy Ann Neurol 58:151–155
Feldkotter M, Schwarzer V, Wirth R, Wienker TF, Wirth B (2002) Quantitative analyses of SMN1 and SMN2 based on real-time lightCycler PCR: fast and highly reliable carrier testing and prediction of severity of spinal muscular atrophy. Am J Hum Genet 70:358–368
Gennarelli M, Lucarelli M, Capon F, Pizzuti A, Merlini L, Angelini C, Novelli G, Dallapiccola B (1995) Survival motor neuron gene transcript analysis in muscles from spinal muscular atrophy patients. Biochem Biophys Res Commun 213:342–348
Great Lake ALS Study Group (2003) A comparison of muscle strength testing techniques in amyotrophic lateral sclerosis Neurology 61:1503–1507
Kaufmann P, Muntoni F (2007) Issues in SMA clinical trial design. The International Coordinating Committee (ICC) for SMA Subcommittee on SMA Clinical Trial Design. Neuromuscul Disord 17:499–505
Lefebvre S, Burglen L, Reboullet S, Clermont O, Burlet P, Viollet L, Benichou B, Cruaud C, Millasseau P, Zeviani M (1995) Identification and characterization of a spinal muscular atrophy-determining gene. Cell 80:155–165
McAndrew PE, Parsons DW, Simard LR, Rochette C, Ray PN, Mendell JR, Prior TW, Burghes AH (1997) Identification of proximal spinal muscular atrophy carriers and patients by analysis of SMNT and SMNC gene copy number. Am J Hum Genet 60:1411–1422
McHorney CA, Ware JE Jr, Lu JF, Sherbourne CD (1994) The MOS 36- item Short-Form Health Survey (SF- 36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups Med Care 32:40–66
Munsat TL, Davies KE (1992) International SMA consortium meeting. (26–28 June 1992, Bonn, Germany) Neuromuscul Disord 2:423–428
O’hagen JM, Glanzman AM, McDermott MP, Ryan PA, Flickinger J, Quigley J, Riley S, Sanborn E, Irvine C, Martens WB, Annis C, Tawil R, Oskoui M, Darras BT, Finkel RS, De V (2007) An expanded version of the Hammersmith Functional Motor Scale for SMA II and III patients. Neuromuscul Disord 17:693–697
Rudnik-Schoneborn S, Hausmanowa- Petrusewicz I, Borkowska J, Zerres K (2001) The predictive value of achieved motor milestones assessed in 441 patients with infantile spinal muscular atrophy types II and III. Eur Neurol 45:174–181
Russman BS, Buncher CR, White M, Samaha FJ, Iannaccone ST (1996) Function changes in spinal muscular atrophy II and III. The DCN/SMA Group. Neurology 47:973–976
Sumner CJ (2006) Therapeutics development for spinal muscular atrophy. NeuroRx 3:235–245
Sumner CJ, Huynh TN, Markowitz JA, Perhac JS, Hill B, Coovert DD, Schussler K, Chen X, Jarecki J, Burghes AH, Taylor JP, Fischbeck KH (2003) Valproic acid increases SMN levels in spinal muscular atrophy patient cells. Ann Neurol 54:647–654
Swoboda KJ, Prior TW, Scott CB, McNaught TP, Wride MC, Reyna SP, Bromberg MB (2005) Natural history of denervation in SMA: relation to age, SMN2 copy number, and function. Ann Neurol 57:704–712
Taylor JE, Thomas NH, Lewis CM, Abbs SJ, Rodrigues NR, Davies KE, Mathew CG (1998) Correlation of SMNt and SMNc gene copy number with age of onset and survival in spinal muscular atrophy. Eur J Hum Genet 6:467–474
Tomaszewicz K, Kang P, Wu BL (2005) Detection of homozygous and heterozygous SMN deletions of spinal muscular atrophy in a single assay with multiplex ligation-dependent probe amplification Beijing Da Xue Xue Bao 37:55–57
Weihl CC, Connolly AM, Pestronk A (2006) Valproate may improve strength and function in patients with type III/ IV spinal muscle atrophy. Neurology 67:500–501
Wirth B, Brichta L, Schrank B, Lochmuller H, Blick S, Baasner A, Heller R (2006) Mildly affected patients with spinal muscular atrophy are partially protected by an increased SMN2 copy number Hum Genet 119:422–428
Zerres K, Rudnik-Schoneborn S (1995) Natural history in proximal spinal muscular atrophy. Clinical analysis of 445 patients and suggestions for a modification of existing classifications. Arch Neurol 52:518–523
Zerres K, Rudnik-Schoneborn S, Forrest E, Lusakowska A, Borkowska J, Hausmanowa-Petrusewicz I (1997) A collaborative study on the natural history of childhood and juvenile onset proximal spinal muscular atrophy (type II and III SMA): 569 patients J Neurol Sci 146:67–72
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Piepers, S., van den Berg, L.H., Brugman, F. et al. A natural history study of late onset spinal muscular atrophy types 3b and 4. J Neurol 255, 1400–1404 (2008). https://doi.org/10.1007/s00415-008-0929-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-008-0929-0