Abstract
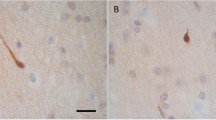
In cases of traumatic brain injury (TBI) in which the patient survived for only a short period of time and was without macroscopic changes at autopsy, it is difficult to diagnose TBI. To detect early diagnostic markers of diffuse axonal injury (DAI), real-time quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) in an experimental head trauma model of rat was chosen. The β-amyloid precursor protein (β-APP) is a well-known diagnostic marker of DAI which can be detected by immunolabeling as early as 1.5 h after injury. β-APP has a binding protein, FE65, which is expressed in the brain of Alzheimer's disease patients along with β-APP, but no involvement with brain injury has been reported. Neuron-specific enolase (NSE) is also a useful marker of DAI. We found that FE65 expression increased dramatically as early as 30 min after injury and decreased after peaking 1 h post-injury, although NSE showed no significant changes. These results suggest that real-time PCR of FE65 mRNA is useful for the diagnosis of DAI in forensic cases.


Similar content being viewed by others
References
Kawarabayashi T, Shoji M, Harigaya Y, Yamaguchi H, Hirai S (1991) Expression of APP in the early stage of brain damage. Brain Res 563:334–338
Nakamura Y, Takeda M, Niigawa H, Hariguchi S, Nishimura T (1992) Amyloid beta-protein precursor deposition in rat hippocampus lesioned by ibotenic acid injection. Neurosci Lett 136:95–98
Otsuka N, Tomonaga M, Ikeda K (1991) Rapid appearance of beta-amyloid precursor protein immunoreactivity in damaged axons and reactive glial cells in rat brain following needle stab injury. Brain Res 568:335–338
Sherriff FE, Bridges LR, Sivaloganathan S (1994) Early detection of axonal injury after human head trauma using immunocytochemistry for β-amyloid precursor protein. Acta Neuropathol 87:55–62
Sherriff FE, Bridges LR, Gentleman SM, Sivaloganathan S, Wilson S (1994) Markers of axonal injury in post mortem human brain. Acta Neuropathol 88:433–439
Gentleman SM, Roberts GW, Gennarelli TA, Maxwell WL, Adams JH, Kerr S, Graham DI (1995) Axonal injury: a universal consequence of fatal closed head injury? Acta Neuropathol 89:537–543
Blumbergs PC, Scott G, Manavis J, Wainwright H, Simpson DA, McLearn AJ (1995) Topography of axonal injury as defined by amyloid precursor protein and the sector scoring method in mild and severe closed head injury. J Neurotrauma 12:565–572
McKenzei KJ, McLellan DR, Gentleman SM, Maxwell WL, Gennarelli TA, Graham DI (1996) Is β-APP a marker of axonal damage in short-surviving head injury? Acta Neuropathol 92:608–613
Gentleman SM, Nash MJ, Sweeting CJ, Grahamn DI, Roberts GW (1993) β-Amyloid precursor protein (β-APP) as a marker for axonal injury after head injury. Neurosci Lett 160:139–144
Cochran E, Bacci B, Chen Y, Patton A, Gambetti P, Autilio-Gambetti L (1991) Amyloid precursor protein and ubiquitin immunoreactivity in dystrophic axons is not unique to Alzheimer's disease. Am J Pathol 139:485–489
Ohgami T, Kitamoto T, Tateishi J (1992) Alzheimer's amyloid precursor protein accumulates within axonal swellings in human brain lesions. Neurosci Lett 17;136:75–78
Koo EH, Sisodia SS, Archer DR, Marin LJ, Weidemann A, Beyreuther K, Fischer P, Masters CL, Price DL (1990) Precursor of amyloid protein in Alzheimer disease undergoes fast anterograde axonal transport. Proc Natl Acad Sci USA 87:1561–1565
Shigematsu K, McGeer PL (1992) Accumulation of amyloid precursor protein in neurons after intraventricular injection of colchicine. Am J Pathol 140:787–794
Adams JH, Doyle D, Graham DI, Lawrence AE, McLellan DR (1984) Diffuse axonal injury in head injuries caused by a fall. Lancet ii:1420–1421
Imajo T, Roessman U (1984) Diffuse axonal injury. Am J Forensic Med Pathol 5:217–222
Blumbergs PC, Jones NR, North JB (1989) Diffuse axonal injury in head trauma. J Neurol Neurosurg Psychiatry 52:838–841
Abou-Hamden A, Blumbergs PC, Scott G, Manavis J, Wainwright H, Jones N, McLean J (1997) Axonal injury in falls. J Neurotrauma 14:699–713
Imajo T, Challencer RC, Roessmann U (1987) Diffuse axonal injury by assault. Am J Forensic Med Pathol 8:217–219
Graham DI, Clark JC, Adams JH, Gennarelli TA (1992) Diffuse axonal injury caused by assault. J Clin Pathol 45:840–841
Strich SJ (1961) Shearing of nerve fibres as a cause of brain damage due to head injury. A pathological study of twenty cases. Lancet ii:443–448
Peerless SJ, Rewcastle NB (1967) Shear injuries of the brain. Can Med Assoc J 96:577–582
Zimmerman RA, Bilaniuk LT, Genneralli T (1978) Computed tomography of shearing injuries of the cerebral white matter. Radiology 127:393–396
Adams JH (1992) Head injury. In: Adams JH, Duchen LW (eds) Greenfields neuropathology, 5th edn. Edward Arnold, London, pp 106–152
Pilz P (1983) Axonal injury in head injury. Acta Neurochir Suppl 32:119–123
Sahuquillo J, Vilalta J, Lamarca J, Rubio E, Rodriguez-Pazos M, Salva JA (1989) Diffuse axonal injury after severe head trauma. Acta Neurochir 101:149–158
Blumbergs PC, Scott G, Manavis J, Wainwright H, Simpson DA, McLean AJ (1994) Staining of amyloid precursor protein to study axonal damage in mild head injury. Lancet 344:1055–1056
Geddes JF, Vowles GH, Beer TW, Ellison DW (1997) The diagnosis of diffuse axonal injury: implications for forensic practice. Neuropathol Appl Neurobiol 23:339–347
Ogata M, Tsuganezawa O (1999) Neuron-specific enolase as an effective immunohistochemical marker for injured axons after fatal brain injury. Int J Legal Med 113:19–25
Russo T, Faraonio R, Minopoli G, De Candia P, Renzis SD, Zambrano N (1998) FE65 and the protein network centered around the cytosolic domain of the Alzheimer's β-amyloid precursor protein. FEBS Lett 434:1–7
Esposito F, Ammendola R, Duilio A, Costanzo F, Giordano M, Zambrano N, D'Agostino P, Russo T, Cimino F (1990) Isolation of cDNA fragments hybridizing to rat brain-specific mRNAs. Dev Neurosci 12:373–381
Duilio A, Zambrano N, Mogavero AR, Ammendola R, Cimino F, Russo T (1991) A rat brain mRNA encoding a transcriptional activator homologous to the DNA binding domain of retroviral integrases. Nucleic Acids Res 19:5269–5274
Sabo SL, Lanier LM, Ikin AF, Khorkova O, Sahasrabudhe S, Greegard P, Buxbaum J (1999) Regulation of β-amyloid secretion by FE65, an amyloid protein precursor-binding protein. J Biol Chem 274:7952–7957
Bressler S, Gray MD, Sopher BL et al. (1996) cDNA cloning and chromosome mapping of the human Fe65 gene; interaction of the conserved cytoplasmic domains of the human β-amyloid precursor protein and its homologues with the mouse Fe65 protein. Hum Mol Genet 5:1589–1598
Delatour B, Mercken L, El Hachimi KH, Colle MA, Pradier L, Duyckaerts C (2001) FE65 in Alzheimer's disease: neuronal distribution and association with neurofibrillary tangles. Am J Pathol 158:1585–1591
Simeone A, Duilio A, Fiore F, Acampora D, De Felice C, Faraonio R, Paolocci F, Cimino F, Russo T (1994) Expression of the neuron-specific FE65 gene marks the development of embryo ganglionic derivatives. Dev Neurosci 16:53–60
Lambert JC, Mann D, Goumidi L et al. (2000) A FE65 polymorphism associated with risk of developing sporadic late-onset Alzheimer's disease but not with Ab loading in brains. Neurosci Lett 293:29–32
Hu Q, Jin LW, Starbuck MY, Martin GM (2000) Broadly altered expression of the mRNA isoforms of FE65, a facilitator of beta amyloidogenesis, in Alzheimer cerebellum and other brain regions. J Neurosci Res 60:73–86
Hu Q, Kukull WA, Bressler SL et al. (1998) The human FE65 gene: genomic structure and an intronic biallelic polymorphism associated with sporadic dementia of the Alzheimer type. Hum Genet 103:295–303
Ermekova KS, Chang A, Zambrano N, De Candia P, Russo T, Sudol M (1998) Proteins implicated in Alzheimer disease. The role of FE65, a new adapter which binds to beta-amyloid precursor protein. Adv Exp Med Biol 446:161–180
Sabo SL, Ikin AF, Buxbaum JD, Greengard P (2001) The Alzheimer amyloid precursor protein (APP) and FE65, an APP-binding protein, regulate cell movement. J Cell Biol 153:1403–1414
Li R, Fujitani N, Jia JT, Kimura H (1998) Immunohistochemical indicators of early brain injury: an experimental study using the fluid-percussion model in cats. Am J Forensic Med Pathol 19:129–136
Marangos PJ, Schmechel DE (1987) Neuron specific enolase, a clinically useful marker for neurons and neuroendocrine cells. Annu Rev Neurosci 10:269–295
Murray GI, Duncan ME, Melvin WT, Fothergill JE (1993) Immunohistochemistry of neurone specific enolase with gamma subunit specific anti-peptide monoclonal antibodies. J Clin Pathol 46:993–996
Schreiber SS, Sun N, Tocco G, Baudry M, DeGiorgio CM (1999) Expression of neuron-specific enolase in adult rat brain following status epilepticus. Exp Neurol 159:329–331
Marangos PJ, Zis AP, Clark RL, Goodwin FK (1978) Neuronal, non-neuronal and hybrid forms of enolase in brain: structural, immunological and functional comparisons. Brain Res 150:117–133
Hatfield RH, McKernan RM (1992) CSF neuron-specific enolase as a quantitative marker of neuronal damage in a rat stroke model. Brain Res 577:249–252
Karkela J, Bock E, Kaukinen S (1993) CSF and serum brain-specific creatine kinase isoenzyme (CK-BB), neuron-specific enolase (NSE) and neural cell adhesion molecule (NCAM) as prognostic markers for hypoxic brain injury after cardiac arrest in man. J Neurol Sci 116:100–109
Maxwell WL, Watt C, Graham DI, Gennarelli TA (1993) Ultrastuctural evidence of axonal shearing as a result of lateral acceleration of the head in non-human primates. Acta Neuropathol 86:136–144
Pettus EH, Christman CW, Giebel Ml, Povlishock JT (1994) Traumatically induced altered membrane permeability: its relationship to traumatically induced reactive axonal change. J Neurotrauma 11:507–522
Graham DI, Maxwell WL, Nicoll JAR (1997) Neurotrauma: axonal damage in short surviving head injury and the influence of apolipoprotein E on outcome. Brain Pathol 7:1285–1288
Gennarelli TA (1996) The spectrum of traumatic axonal injury. Neuropathol Appl Neurobiol 22:509–513
Maxwell WL, Povlishock JT, Graham DI (1997) A mechanistic analysis of nondisruptive axonal injury: a review. J Neurotrauma 14:419–440
Povlishock JT, Christman CW (1995) The pathobiology of traumatically induced axonal injury in animals and humans: a review of current thoughts. J Neurotrauma 12:555–564
Heid CA, Stevens J, Livak KJ, Williams PM (1996) Real time quantitative PCR. Genome Res 6:986–994
Gibson UE, Heid CA, Williams PM (1996) A novel method for real time quantitative RT-PCR. Genome Res 6:995–1001
Aldape K, Ginzinger DG, Godfrey TE (2002) Real-time quantitative polymerase chain reaction: a potential tool for genetic analysis in neuropathology. Brain Pathol 12:54–66
Wang X, Li X, Currie RW, Willette RN, Barone FC, Feuerstein GZ (2000) Application of real-time polymerase chain reaction to quantitate induced expression of interleukin-1beta mRNA in ischemic brain tolerance. J Neurosci Res 59:238–246
Li X, Wang X (2000) Application of real-time polymerase chain reaction for the quantitation of interleukin-1beta mRNA upregulation in brain ischemic tolerance. Brain Res Brain Res Protoc 5:211–217
Tolentino PJ, DeFord SM, Notterpek L, Glenn CC, Pike BR, Wan KK, Hayes RL (2002) Up-regulation of tissue-type transglutaminase after traumatic brain injury. J Neurochem 80:579–588
Marmarou A, Foda MA, Brink W van den, Campbell J, Kita H, Demetriadou K (1994) A new model of diffuse brain injury in rats. Part I: Pathophysiology and biomechanics. J Neurosurg 80:291–300
Hausmann R, Kaiser A, Lang C, Bohnert M, Betz P (1999) A quantitative immunohistochemical study on the time-dependent course of acute inflammatory cellular response to human brain injury. Int J Legal Med 112:227–232
Hausmann R, Riess R, Fieguth A, Betz P (2000) Immunohistochemical investigations on the course of astroglial GFAP expression following human brain injury. Int J Legal Med 113:70–75
Hausmann R, Betz P (2000) The time course of the vascular response to human brain injury—an immunohistochemical study. Int J Legal Med 113:288–292
Hausmann R, Betz P (2001) Course of glial immunoreactivity for vimentin, tenascin and alpha1-antichymotrypsin after traumatic injury to human brain. Int J Legal Med 114:338–342
Orihara Y, Ikematsu K, Tsuda R, Nakasono I (2001) Induction of nitric oxide synthase by traumatic brain injury. Forensic Sci Int 123:142–149
Orihara Y (2000) Forensic pathological significance of iNOS with regard to brain and myocardial damage (in Japanese). Nippon Hoigaku Zasshi 54:361–366
Orihara Y, Nakasono I (2002) Induction of apolipoprotein E after traumatic brain injury in forensic autopsy cases.Int J Legal Med 116:92–98
Nogami M, Takatsu A, Ishiyama I (1998) Immunohistochemical study of neuron-specific enolase in human brains from forensic autopsies. Forensic Sci Int 94:97–109
Gotohda T, Kubo S, Kitamura O, Ishigami A, Tokunaga I (2001) Neuronal changes in the arcuate and hypoglossal nuclei of brain stem induced by head injury. Int J Legal Med 115:121–127
Kita T, Tanaka T, Tanaka N, Kinoshita Y (2000) The role of tumor necrosis factor-α in diffuse axonal injury following fluid-percussive brain injury in rats. Int J Legal Med 113:221–228
Kita T, Liu L, Tanaka N, Kinoshita Y (1997) The expression of tumor necrosis factor-α in the rat brain after fluid percussive injury. Int J Legal Med 110:305–311
Stahel PF, Kossmann T, Morganti-Kossmann MC, Hans VH, Barnum SR (1997) Experimental diffuse axonal injury induces enhanced neuronal C5a receptor mRNA expression in rats. Brain Res Mol Brain Res 50:205–212
Masumura M, Hata R, Uramoto H, Murayama N, Ohno T, Sawada T (2000) Altered expression of amyloid precursors proteins after traumatic brain injury in rats: in situ hybridization and immunohistochemical study. J Neurotrauma 17:123–134
Bodjarian N, Jamali S, Boisset N, Tadie M (1997) Strong expression of GFAP mRNA in rat hippocampus after a closed-head injury. Neuroreport 8:3951–3956
Fan L, Young PR, Barone FC, Feuerstein GZ, Smith DH, McIntosh TK (1995) Experimental brain injury induces expression of interleukin-1 beta mRNA in the rat brain. Brain Res Mol Brain Res 30:125–130
Ishida K, Zhu BL, Maeda H (2000) Novel approach to quantitative reverse transcription PCR assay of mRNA component in autopsy material using the TaqMan fluorogenic detection system: dynamics of pulmonary surfactant apoprotein A. Forensic Sci Int 113:127–131
Marmarou A, Shima K (1990) Comparative studies of edema produced by fluid percussion injury with lateral and central modes of injury in cats. Adv Neurol 52:233–236
Schmidt RH, Grady MS (1993) Regional patterns of blood-brain barrier breakdown following central and lateral fluid percussion injury in rodents. J Neurotrauma 10:415–430
Shima K, Marmarou A (1991) Evaluation of brain-stem dysfunction following severe fluid-percussion head injury to the cat. J Neurosurg 74:270–277
Thibault LE, Meaney DF, Anderson BJ, Marmarou A (1992) Biomechanical aspects of a fluid percussion model of brain injury. J Neurotrauma 9:311–322
McIntosh TK, Vink R, Noble L, Yamakami I, Fernyak S, Soares H, Faden AL (1989) Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience 28:233–244
Lighthall JW (1988) Controlled cortical impact: a new experimental brain injury model. J Neurotrauma 5:1–15
Dixon CE, Clifton GL, Lighthall JW, Yaghmai AA, Hayes RL (1991) A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods 39:253–262
Gennarelli TA, Thibault LE, Adams JH, Graham DI, Thompson CJ, Marcincin RP (1982) Diffuse axonal injury and traumatic coma in the primate. Ann Neurol 12:564–574
Feeney DM, Boyeson MG, Linn RT, Murray HM, Dail WG (1981) Responses to cortical injury: I. Methodology and local effects of contusions in the rat. Brain Res 211:67–77
Tornheim PA, Liwnicz BH, Hirsch CS, Brown DL, McLaurin RL (1983) Acute responses to blunt head trauma. Experimental model and gross pathology. J Neurosurg 59:431–438
Foda MA, Marmarou A (1994) A new model of diffuse brain injury in rats. Part II: Morphological characterization. J Neurosurg 80:301–313
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iino, M., Nakatome, M., Ogura, Y. et al. Real-time PCR quantitation of FE65 a β-amyloid precursor protein-binding protein after traumatic brain injury in rats. Int J Legal Med 117, 153–159 (2003). https://doi.org/10.1007/s00414-003-0370-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-003-0370-y