Abstract
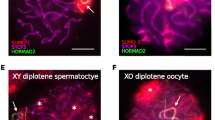
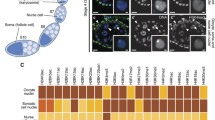
In the XY pachytene spermatocyte, the sex chromosomes do not synapse except for the pseudoautosomal region and become transcriptionally silenced. It has been suggested that the meiotic silencing of unsynapsed chromatin (MSUC) also occurs in oocytes. In the XY sex-reversed female mouse, the sex chromosomes fail to pair in the majority of oocytes and a greater number of oocytes are eliminated during the meiotic prophase compared to the XX female. Yet, many XY oocytes survive to reach the second meiotic metaphase. The goal of our current study was to determine whether the single X chromosome shows the characteristics of asynapsis and meiotic silencing in a proportion of XY oocytes, which can explain the survival of the remaining oocytes. We first examined the accumulation of markers associated with asynapsis or transcriptional silencing, i.e., BRCA1, γH2AX, H3K9me3, and H3K27me3, at the single X chromosome in the XY oocyte. We found that γH2AX and BRCA1 were enriched on the single X chromosome whereas H3K9me3 was not, and H3K27me3 was enriched at all chromosomes in the majority of XY oocytes. We next examined the meiotic silencing of the single X chromosome using enrichment of the X-encoded ATRX protein. On average, ATRX enrichment was lower in XY oocytes than in XX oocytes as expected from its half gene dosage. However, the intensity of ATRX staining in XY oocytes harboring γH2AX domains showed a remarkable heterogeneity. We conclude that MSUC occurs with varying consequences, resulting in a heterogeneous population of oocytes with respect to protein enrichment in the XY female mouse.








Similar content being viewed by others
References
Alton M, Lau MP, Villemure M, Taketo T (2008) The behavior of the X- and Y-chromosomes in the oocyte during meiotic prophase in the B6.YTIR sex-reversed mouse ovary. Reproduction 135:241–252
Amleh A, Smith L, Chen H-Y, Taketo T (2000) Both nuclear and cytoplasmic components are defective in oocytes of the B6.YTIR sex-reversed female mouse. Dev Biol 219:277–286
Baarends WM, Wassenaar E, van der Laan R, Hoogerbrugge J, Sleddens-Linkels E, Hoejimakers JHJ, de Boer P, Grootegoed JA (2005) Silencing of unpaired chromatin and histone H2A ubiquitination in mammalian meiosis. Mol Cell Biol 25:1041–1053
Baker TG (1963) A quantitative and cytological study of germ cells in human ovaries. Proc R Soc Lond B158:417–433
Bakken AH, McClanahan M (1978) Patterns of RNA synthesis in early meiotic prophase oocytes from fetal mouse ovaries. Chromosoma 67:21–40
Baumann C, Schmidtmann A, Muegge K, De La Fuente R (2008) Association of ATRX with pericentric heterochromatin and the Y chromosome of neonatal mouse spermatogonia. BMC Mol Biol 9(29)
Bernstein E, Duncan EM, Masui O, Gil J, Heard E, Allis CD (2006) Mouse polycomb proteins bind differentially to methylated histone H3 and RNA and are enriched in facultative heterochromatin. Mol Cell Biol 26:2560–2569
Burgoyne PS, Baker TG (1985) Perinatal oocyte loss in XO mice and its implications for the aetiology of gonadal dysgenesis in XO women. J Reprod Fert 75:633–645
Burgoyne PS, Mahadevaiah SK, Sutcliffe MJ, Palmer SJ (1992) Fertility in mice requires X-Y pairing and a Y-chromosomal "spermiogenesis" gene mapping to the long arm. Cell 71:391–398
de la Fuente R, Viveiros MM, Wigglesworth K, Eppig JJ (2004) ATRX, a member of the SNF2 family of helicase-ATPase, is required for chromosome alignment and meiotic spindle organization in metaphase II stage mouse oocyte. Dev Biol 272:1–14
de Vries SS, Baart EB, Dekker M, Siezen A, de Rooij DG, de Boer P, te Riele H (1999) Mouse MutS-like protein Msh5 is required for proper chromosome synapsis in male and female meiosis. Genes Dev 13:523–531
Dobson MJ, Pearlman RE, Karaiskakis A, Spyropoulos B, Moens PB (1994) Synaptonemal complex proteins: occurrence, epitope mapping and chromosome disjunction. J Cell Sci 107:2749–2760
Fernandez-Capetillo O, Mahadevaiah SK, Celeste A, Romanienko PJ, Camerini-Otero RD, Bonner WM, Manova K, Burgoyne P, Nussenzweig A (2003) H2AX is required for chromatin remodeling and inactivation of sex chromosomes in male mouse meiosis. Dev Cell 4:497–508
Gibbons RJ, Bachoo S, Picketts DJ, Aftimos S, Asenbauer B, Bergoffen J, Berry SA, Dahl N, Fryer A, Keppler K, Kurosawa K, Levin ML, Masuno M, Neri G, Pierpont ME, Slaney SF, Higgs DR (1997) Mutations in transcriptional regulator ATRX establish the functional significance of a PHD-like domain. Nat Genet 17:146–148
Guioli S, Lovell-Badge R, Turner JMA (2012) Error-prone ZW pairing and no evidence for meiotic sex chromosome inactivation in the chicken germ line. PLoS Genet 8:e1002560
Hajkova P, Ancelin K, Waldmann T, Lacoste N, Lange UC, Cesari F, Lee C, Almouzni G, Schneider R, Surani MA (2008) Chromatin dynamics during epigenetic reprogramming in the mouse germ line. Nature 452:877–881
Homolka D, Jansa P, Forejt J (2012) Genetically enhanced asynapsis of autosomal chromatin promotes transcriptional dysregulation and meiotic failure. Chromosoma 121:91–104
Ichijima Y, Ichijima M, Lou Z, Nussenzweig A, Daniel Camerini-Otero R, Chen J, Andreassen PR, Namekawa SH (2011) MDC1 directs chromosome-wide silencing of the sex chromosomes in male germ cells. Genes Dev 25:959–971
Kim Y, Kobayashi A, Sekido R, DiNapoli L, Brennan J, Chaboissier M-C, Poulat F, Behringer RR, Lovell-Badge R, Capel B (2006) Fgf9 and Wnt4 act as antagonistic signals to regulate mammalian sex determination. PLos Biol 4:e187
Kota SK, Feil R (2010) Epigenetic transitions in germ cell development and meiosis. Dev Cell 19:675–686
Kouznetsova A, Wang H, Bellani M, Camerini-Otero RD, Jessberger R, Hoog C (2009) BRCA1-mediated chromatin silencing is limited to oocytes with a small number of asynapsed chromosomes. J Cell Sci 122:2446–2452
Kralewski M, Benavente R (1997) XY body formation during rat spermatogenesis: an immuocytochemical study using antibodies against XY body-associated proteins. Chromosoma 106:304–307
Lawson KA, Dunn NR, Roelen BAJ, Zeinstra LM, Davis AM, Wright CVE, Korving JPWFM, Hogan BLM (1999) Bmp4 is required for the generation of primordial germ cells in the mouse embryos. Genes Dev 13:424–436
Lyon MF, Hawker SG (1973) Reproductive lifespan in irradiated and unirradiated chromosomally XO mice. Genet Res Camb 21:185–194
Maatouk DM, DiNapoli L, Alvers A, Parker KL, Taketo MM, Capel B (2008) Stabilization of β-catenin in XY gonads causes male-to-female sex-reversal. Hum Mol Genet 17:2949–2955
Mahadevaiah SK, Turner JMA, Baudat F, Rogakou EP, de Boer P, Blanco-Rodriguez J, Jasin M, Keeney S, Bonner WM, Burgoyne PS (2001) Recombnational DNA double-strand breaks in mice precede synapsis. Nat Genet 27:271–276
Mahadevaiah SK, Bourc’his D, de Rooij DG, Bestor TH, Turner JMA, Burgoyne PS (2008) Extensive meiotic asynapsis in mice antagonises meiotic silencing of unsynapsed chromatin and consequently disrupts meiotic sex chromosome inactivation. J Cell Biol 182:263–276
McClellan KA, Gosden R, Taketo T (2003) Continuous loss of oocytes throughout meiotic prophase in the normal mouse ovary. Dev Biol 258:334–348
Mittwoch U, Mahadevaiah SK (1992) Unpaired chromosomes at meiosis: cause or effect of gametogenic insufficiency? Cytogenet Cell Genet 59:274–279
Moens PB, Chen DJ, Shen Z, Kolas N, Tarsounas M, Heng HHQ, Spyropoulos B (1997) Rad51 immunocytology in rat and mouse spermatocytes and oocytes. Chromosoma 106:207–215
Moens PB, Marcon E, Shore JS, Kochakpour N, Spyropoulos B (2007) Initiation and resolution of interhomolog connections: crossover and non-crossover sites along mouse synaptonemal complexes. J Cell Sci 120:1017–1027
Molyneaux KA, Stallock J, Schaible K, Wylie C (2001) Time-lapse analysis of living mouse germ cell migration. Dev Biol 240:488–498
Nagamine CM, Taketo T, Koo GC (1987) Studies on the genetics of tda-1 XY sex reversal in the mouse. Differentiation 33:223–231
Obata Y, Villemure M, Kono T, Taketo T (2008) Transmission of Y chromosomes from XY female mice was made possible by the replacement of cytoplasm during oocyte maturation. Proc Natl Acad Sci USA 105:13918–13923
Ooi SL, Henikoff S (2007) Germline histone dynamics and epigenetics. Curr Op Cell Biol 19:257–265
Ottolenghi C, Omari S, Garcia-Ortiz JE, Uda M, Crisponi L, Forabosco A, Pilia G, Schlessinger D (2005) Foxl2 is required for commitment to ovary differentiation. Hum Mol Genet 14:2053–2062
Page J, De La Fuente R, Manterola M, Parra MT, Viera A, Berríos S, Fernández-Donoso R, Rufas JS (2012) Inactivation or non-reactivation: what accounts better for the silence of sex chromosomes during mammalian male meiosis? Chromosoma 121:307–326
Petukhova GV, Romanienko PJ, Camerini-Otero RD (2003) The Hop2 protein has a direct role in promoting interhomolog interactions during mouse meiosis. Dev Cell 5:927–936
Royo H, Polikiewicz G, Mahadevaiah SK, Prosser H, Mitchell M, Bradley A, de Rooij DG, Burgoyne PS, Turner JMA (2010) Evidence that meiotic sex chromosome inactivation is essential for male fertility. Curr Biol 20:2117–2123
Saferali A, Berlivet S, Schimenti J, Bartolomei MS, Taketo T, Naumova AK (2010) Defective imprint resetting in carriers of Robertsonian translocation Rb (8.12). Mamm Genome 21:377–387
Sciurano R, Rahn M, Rey-Valzacchi G, Solari AJ (2007) The asynaptic chromatin in spermatocytes of an location carriers contains the histone variant γ-H2AX and associates with the XY body. Hum Reprod 22:142–150
Scully R, Chen J, Plug A, Xiao Y, Weaver D, Feunteun J, Ashley T, Livingston DM (1997) Association of BRCA1 with Rad51 in mitotic and meiotic cells. Cell 88:265–275
Silva J, Mak W, Zvetkova I, Appanah R, Nesterova TB, Webster Z, Peters AHFM, Jenuwein T, Otte AP, Brockdorff N (2003) Establishment of histone H3 methylation on the inactive X chromosome requires transient recruitment of Eed-Enx1 polycomb group complexes. Dev Cell 4:481–495
Solari AJ (1980) Synaptonemal complexes and associated structures in microspread human spermatocytes. Chromosoma 81:315–337
Speed RM (1986) Oocyte development in XO foetuses of man and mouse: the possible role of heterologous X-chromosome pairing in germ cell survival. Chromosoma 94:115–124
Tachibana M, Nozaki M, Takeda N, Shinkai Y (2007) Functional dynamics of H3K9 methylation during meiotic prophase progression. EMBO J 26:3346–3356
Taketo T (2012) Microspread oocyte preparations for the analysis of meiotic prophase progression with improved recovery by cytospin centrifugation. Meth Mol Biol 825:173–181
Taketo-Hosotani T, Nishioka Y, Nagamine C, Villalpando I, Merchant-Larios H (1989) Development and fertility of ovaries in the B6.YDOM sex-reversed female mouse. Development 107:95–105
Tam PPL, Zhou SX (1996) The allocation of epiblast cells to ectodermal and germ-line lineages is influenced by the position of the cells in the gastrulating mouse embryo. Dev Biol 178:124–132
Turner JMA, Mahadevaiah SK, Benavente R, Offenberg HH, Heyting C, Burgoyne PS (2000) Analysis of male meiotic "sex body" proteins during XY female meiosis provides new insights into their functions. Chromosoma 109:426–432
Turner JMA, Aprelikova O, Xu X, Wang R, Kim S, Chandramouli GVR, Barrett JC, Burgoyne PS, Deng C-X (2004) BRCA1, histone H2AX phosphorylation, and male meiotic sex chromosome inactivation. Curr Biol 14:2135–2142
Turner JMA, Mahadevaiah SK, Fernandes-Capetillo O, Nussenzweig A, Xu X, Deng C-X, Burgoyne PS (2005) Silencing of unsynapsed meiotic chromosomes in the mouse. Nat Genet 37:41–47
Turner JMA, Mahadevaiah SK, Ellis PJI, Mitchell MJ, Burgoyne PS (2006) Pachytene asynapsis drives meiotic sex chromosome inactivation and leads to substantial postmeiotic repression in spermatids. Dev Cell 10:521–529
van der Heijden GW, Derijck AAHA, Posfai E, Geile M, Pelczar P, Ramos LWDG, van der Vlag J, Peters AHFM, de Boer P (2007) Chromosome-wide nucleosome replacement and H3.3 incorporation during mammalian meiotic sex chromosome inactivation. Nat Genet 39:251–258
Xu B-Z, Obata Y, Cao F, Taketo T (2012) The presence of the Y-chromosome, not the absence of the second X-chromosome, alters the mRNAs stored in the fully grown XY mouse oocyte. PLoS One 7:e40481
Acknowledgments
We are grateful for the gift of antibodies to Drs. P. Moens (deceased) and S. Namekawa. This work was supported by funds from the Natural Sciences and Engineering Research Council of Canada Discovery Grants program (to AN and TT).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Taketo, T., Naumova, A.K. Oocyte heterogeneity with respect to the meiotic silencing of unsynapsed X chromosomes in the XY female mouse. Chromosoma 122, 337–349 (2013). https://doi.org/10.1007/s00412-013-0415-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00412-013-0415-z