Abstract
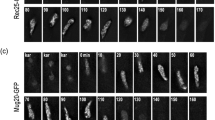
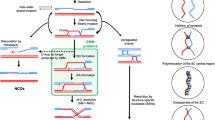
In the fission yeast, Schizosaccharomyces pombe, synaptonemal complexes (SCs) are not formed during meiotic prophase. However, structures resembling the axial elements of SCs, the so-called linear elements (LinEs) appear. By in situ immunostaining, we found Pmt3 (S. pombe's SUMO protein) transiently along LinEs, suggesting that SUMOylation of some component(s) of LinEs occurs during meiosis. Mutation of the SUMO ligase Pli1 caused aberrant LinE formation and reduced genetic recombination indicating a role for SUMOylation of LinEs for the regulation of meiotic recombination. Western blot analysis of TAP-tagged Rec10 demonstrated that there is a Pli1-dependent posttranslational modification of this protein, which is a major LinE component and a distant homolog of the SC protein Red1. Mass spectrometry (MS) analysis revealed that Rec10 is both phosphorylated and ubiquitylated, but no evidence for SUMOylation of Rec10 was found. These findings indicate that the regulation of LinE and Rec10 function is modulated by Pli1-dependent SUMOylation of LinE protein(s) which directly or indirectly regulates Rec10 modification. On the side, MS analysis confirmed the interaction of Rec10 with the known LinE components Rec25, Rec27, and Hop1 and identified the meiotically upregulated protein Mug20 as a novel putative LinE-associated protein.




Similar content being viewed by others
References
Agarwal S, Roeder GS (2000) Zip3 provides a link between recombination enzymes and synaptonemal complex proteins. Cell 102:245–255
Bähler J, Wyler T, Loidl J, Kohli J (1993) Unusual nuclear structures in meiotic prophase of fission yeast: a cytological analysis. J Cell Biol 121:241–256
Bähler J, Wu JQ, Longtine MS, Shah NG, Mckenzie A, Steever AB, Wach A, Philippsen P, Pringle JR (1998) Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14:943–951
Carballo JA, Johnson AL, Sedgwick SG, Cha RS (2008) Phosphorylation of the axial element protein Hop1 by Mec1/Tel1 ensures meiotic interhomolog recombination. Cell 132:758–770
Cervantes MD, Farah JA, Smith GR (2000) Meiotic DNA breaks associated with recombination in S. pombe. Mol Cell 5:883–888
Cheng C-H, Lo Y-H, Liang S-S, Ti S-C, Lin F-M, Yeh C-H, Huang H-Y, Wang T-F (2006) SUMO modifications control assembly of synaptonemal complex and polycomplex in meiosis of Saccharomyces cerevisiae. Genes Dev 20:2067–2081
Cheng C-H, Lin F-M, Lo Y-H, Wang T-F (2007) Tying SUMO modifications to dynamic behaviors of chromosomes during meiotic prophase of Saccharomyces cerevisiae. J Biomed Sci 14:481–490
Davis L, Rozalén AE, Moreno S, Smith GR, Martín-Castellanos C (2008) Rec25 and Rec27, novel linear-element components, link cohesin to meiotic DNA breakage and recombination. Curr Biol 18:849–854
Ding D-Q, Sakurai N, Katou Y, Itoh T, Shirahige K, Haraguchi T, Hiraoka Y (2006) Meiotic cohesins modulate chromosome compaction during meiotic prophase in fission yeast. J Cell Biol 174:499–508
Ellermeier C, Smith GR (2005) Cohesins are required for meiotic DNA breakage and recombination in Schizosaccharomyces pombe. Proc Natl Acad Sci USA 102:10952–10957
Forsburg SL, Rhind N (2006) Basic methods for fission yeast. Yeast 23:173–183
Gregan J, Rabitsch PK, Sakem B, Csutak O, Latypov V, Lehmann E, Kohli J, Nasmyth K (2005) Novel genes required for meiotic chromosome segregation are identified by a high-throughput knockout screen in fission yeast. Curr Biol 15:1663–1669
Gutz H, Heslot H, Leupold U, Loprieno N (1974) Schizosaccharomyces pombe. Handbook of Genetics. pp. 395–446
Henderson KA, Keeney S (2004) Tying synaptonemal complex initiation to the formation and programmed repair of DNA double-strand breaks. Proc Natl Acad Sci USA 101:4519–4524
Hirata A, Tanaka K (1982) Nuclear behavior during conjugation and meiosis in the fission yeast Schizosaccharomyces pombe. J Gen Appl Microbiol 28:263–274
Ho JCY, Warr NJ, Shimizu H, Watts FZ (2001) SUMO modification of Rad22, the Schizosaccharomyces pombe homologue of the recombination protein Rad52. Nucleic Acids Res 29:4179–4186
Hooker GW, Roeder GS (2006) A role for SUMO in meiotic chromosome synapsis. Curr Biol 16:1238–1243
Keller A, Nesvizhskii AI, Kolker E, Aebersold R (2002) Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem 74:5383–5392
Klein F, Laroche T, Cardenas ME, Hofmann JFX, Schweizer D, Gasser SM (1992) Localization of RAP1 and topoisomerase II in nuclei and meiotic chromosomes of yeast. J Cell Biol 117:935–948
Klein F, Mahr P, Galova M, Buonomo SBC, Michaelis C, Nairz K, Nasmyth K (1999) A central role for cohesins in sister chromatid cohesion, formation of axial elements, and recombination during yeast meiosis. Cell 98:91–103
Koshiyama A, Hamada FN, Namekawa SH, Iwabata K, Sugawara H, Sakamoto A, Ishizaki T, Sakaguchi K (2006) Sumoylation of a meiosis-specific RecA homolog, Lim15/Dmc1, via interaction with the small ubiquitin-related modifier (SUMO)-conjugating enzyme Ubc9. FEBS J 273:4003–4012
Loidl J (2006) S. pombe linear elements: the modest cousins of synaptonemal complexes. Chromosoma 115:260–271
Lorenz A, Wells JL, Pryce DW, Novatchkova M, Eisenhaber F, McFarlane RJ, Loidl J (2004) S. pombe meiotic linear elements contain proteins related to synaptonemal complex components. J Cell Sci 117:3343–3351
Lorenz A, Estreicher A, Kohli J, Loidl J (2006) Meiotic recombination proteins localize to linear elements in Schizosaccharomyces pombe. Chromosoma 115:330–340
Martín-Castellanos C, Blanco M, Rozalén AE, Pérez-Hidalgo L, García AI, Conde F, Mata J, Ellermeier C, Davis L, San Segundo PA, Smith GR, Moreno S (2005) A large-scale screen in S. pombe identifies seven novel genes required for critical meiotic events. Curr Biol 15:2056–2062
Mata J, Lyne R, Burns G, Bähler J (2002) The transcriptional program of meiosis and sporulation in fission yeast. Nat Genet 32:143–147
Molnar M, Bähler J, Sipiczki M, Kohli M (1995) The rec8 gene of Schizosaccharomyces pombe is involved in linear element formation, chromosome pairing and sister-chromatid cohesion during meiosis. Genetics 141:61–73
Molnar M, Doll E, Yamamoto A, Hiraoka Y, Kohli J (2003) Linear element formation and their role in meiotic sister chromatid cohesion and chromosome pairing. J Cell Sci 116:1719–1731
Munz P (1994) An analysis of interference in the fission yeast Schizosaccharomyces pombe. Genetics 137:701–707
Nesvizhskii AI, Keller A, Kolker E, Aebersold R (2003) A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem 75:4646–4658
Niu H, Job E, Park C, Moazed D, Gygi SP, Hollingsworth NM (2007) Mek1 kinase is regulated to suppress double-strand break repair between sister chromatids during budding yeast meiosis. Mol Cell Biol 27:5456–5467
Octobre G, Lorenz A, Loidl J, Kohli J (2008) The Rad52 homologs Rad22 and Rti1 of Schizosaccharomyces pombe are not essential for meiotic interhomolog recombination, but are required for meiotic intrachromosomal recombination and mating-type-related DNA repair. Genetics 178:2399–2412
Olson LW, Edén U, Mitani ME, Egel R (1978) Asynaptic meiosis in fission yeast? Hereditas 89:189–199
Osman F, Dixon J, Doe CL, Whitby MC (2003) Generating crossovers by resolution of nicked Holliday junctions: a role for Mus81-Eme1 in meiosis. Mol Cell 12:761–774
Pryce DW, McFarlane RJ (2009) The meiotic recombination hot spots of Schizosaccharomyces pombe. Genome Dyn Stab 5:1–13
Pryce DW, Lorenz A, Smirnova JB, Loidl J, McFarlane RJ (2005) Differential activation of M26-containing meiotic recombination hot spots in Schizosaccharomyces pombe. Genetics 170:95–106
Rigaut G, Shevchenko A, Rutz B, Wilm M, Mann M, Séraphin B (1999) A generic protein purification method for protein complex characterization and proteome exploration. Nat Biotech 17:1030–1032
Sacher M, Pfander B, Hoege C, Jentsch S (2006) Control of Rad52 recombination activity by double-strand break-induced SUMO modification. Nature Cell Biol 8:1284–1290
Sato M, Dhut S, Toda T (2005) New drug-resistant cassettes for gene disruption and epitope tagging in Schizosaccharomyces pombe. Yeast 22:583–591
Shevchenko A, Roguev A, Schaft D, Buchanan L, Habermann B, Sakalar C, Thomas H, Krogan NJ, Shevchenko A, Stewart AF (2008) Chromatin Central: towards the comparative proteome by accurate mapping of the yeast proteomic environment. Genome Biol 9:R167
Spink K, Ho JCY, Tanaka K, Watts FZ, Chambers A (2005) The telomere-binding protein Taz1p as a target for modification by a SUMO-1 homologue in fission yeast. Biochem Genet 43:103–117
Tasto JJ, Carnahan RH, McDonald WH, Gould KL (2001) Vectors and gene targeting modules for tandem affinity purification in Schizosaccharomyces pombe. Yeast 18:657–662
Watts FZ (2007) The role of SUMO in chromosome segregation. Chromosoma 116:15–20
Watts FZ, Skilton A, Ho JCY, Boyd LK, Trickey MAM, Gardner L, Ogi FX, Outwin EA (2007) The role of Schizosaccharomyces pombe SUMO ligases in genome stability. Biochem Soc Trans 35:1379–1384
Wells JL, Pryce DW, Estreicher A, Loidl J, McFarlane RJ (2006) Linear element-independent meiotic recombination in Schizosaccharomyces pombe. Genetics 174:1105–1114
Zickler D, Kleckner N (1999) Meiotic chromosomes: integrating structure and function. Annu Rev Genet 33:603–754
Acknowledgments
The meiotic cDNA library was kindly provided by Hiroshi Nojima (Osaka University) and strains and plasmids by Kathy Gould (Vanderbilt University, Nashville), Alexander Lorenz (University of Oxford), Cristina Martín-Castellanos (Universidad de Salamanca), Gerald Smith (Fred Hutchinson Cancer Research Center, Seattle), Takashi Toda (Cancer Research UK, London), and Matthew Whitby (University of Oxford). We gratefully acknowledge the help of Lubos Cipak and the Ammerer lab (MFPL Vienna) with biochemical experiments and Maria Novatchkova (IMP Vienna) with bioinformatics, and we are grateful to Hubert Renauld (Vienna Medical University) for valuable suggestions. This work was supported by grant no. P18186 from the Austrian Science Fund (FWF) and by grant no. I031-B from the University of Vienna.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Cohen
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Table S1
Strain list (DOC 61 kb)
Supplemental Fig. S2
Treatment with calf intestinal phosphatase (CIP) does not notably reduce the amount of high-molecular-weight Rec10 species, suggesting that the slower migrating forms of Rec10 are not (only) due to phosphorylation. As a positive control for CIP activity, the size reduction of S. cerevisiae Mks1 protein in the presence of CIP is shown. TCA protein extracts were prepared as described for whole-protein isolation (in the “Materials and methods” section) except that the TCA pellets were neutralized by washing with 2M Tris pH8.0 and resuspended in 10 mM Tris buffer pH8.0. The whole proteins were incubated with or without CIP (Roche) for 1 h at 37°C in the corresponding buffer supplied by manufacturer. The procedure was described by Sekito et al. (2002, Mol. Biol. Cell 13:795-804). S. cerevisiae Mks1 is known to be phosphorylated (Sekito et al. 2002; Ferreira Júnior et al. 2005; Gene 354:2-8PSY142). Cells were grown in YPD medium and 3× HA- and 12× His-tagged Mks1 was detected on the Western blot with anti-HA antiserum (DOC 134 kb)
Supplemental S3
(PPT 613 kb)
Rights and permissions
About this article
Cite this article
Spirek, M., Estreicher, A., Csaszar, E. et al. SUMOylation is required for normal development of linear elements and wild-type meiotic recombination in Schizosaccharomyces pombe . Chromosoma 119, 59–72 (2010). https://doi.org/10.1007/s00412-009-0241-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00412-009-0241-5