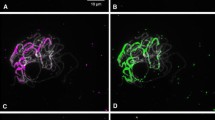
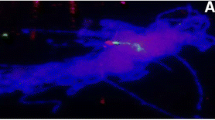
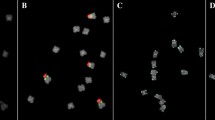
Abstract
Although the monomer size, nucleotide sequence, abundance and species distribution of tandemly organized DNA families are well characterized, little is known about the internal structure of tandem arrays, including total arrays size and the pattern of monomers distribution. Using our rye specific probes, pSc200 and pSc250, we addressed these issues for telomere associated rye heterochromatin where these families are very abundant. Fluorescence in situ hybridization (FISH) on meiotic chromosomes revealed a specific mosaic arrangement of domains for each chromosome arm where either pSc200 or pSc250 predominates without any obvious tendency in order and size of domains. DNA of rye-wheat monosomic additions studied by pulse field gel electrophoresis produced a unique overall blot hybridization display for each of the rye chromosomes. The FISH signals on DNA fibres showed multiple monomer arrangement patterns of both repetitive families as well as of the Arabidopsis-type telomere repeat. The majority of the arrays consisted of the monomers of both families in different patterns separated by spacers. The primary structure of some spacer sequences revealed scrambled regions of similarity to various known repetitive elements. This level of complexity in the long-range organization of tandem arrays has not been previously reported for any plant species. The various patterns of internal structure of the tandem arrays are likely to have resulted from evolutionary interplay, array homogenization and the generation of heterogeneity mediated by double-strand breaks and associated repair mechanisms.




Similar content being viewed by others
References
Alexandrov I, Kazakov A, Tumeneva I, Shepelev V, Yurov Y (2002) Alpha-satellite DNA of primates: old and new families. Chromosoma 110:253–266
Ananiev EV, Phillips RL, Rines HW (1998a) Chromosome-specific molecular organization of maize (Zea mays L.) centromeric regions. Proc Natl Acad Sci USA 95:13073–13078
Ananiev EV, Phillips RL, Rines HW (1998b) Complex structure of knob DNA on maize chromosome 9: retrotransposon invasion into heterochromatin. Genetics 149:2025–2037
Appels R, Dennis ES, Smith DR, Peacock WJ (1981) Two repeated DNA sequences from the heterochromatic regions of rye Secale cereale chromosomes. Chromosoma 84:265–277
Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K (1987) Current protocols in molecular biology. Greene/Wiley Interscience, New York
Bedbrook JR, Jones J, O’Dell M, Tompson R, Flavell R (1980) A molecular description of telomeric heterochromatin in Secale species. Cell 19:545–560
Britten RJ, Kohn DE (1968) Repeated sequences in DNA. Science 161:529–541
Charlesworth B, Sniegowski P, Stephan W (1994) The evolutionary dynamics of repetitive DNA in eukaryotes. Nature 371:215–220
Cheng Z, Buell CR, Wing RA, Gu M, Jiang J (2001) Toward a cytological characterization of the rice genome. Genome Res 11:2133–2141
Cheng Z, Dong F, Langdon T, Ouyang S, Buell CR, Gu M, Blattner FR, Jiang J (2002) Functional rice centromeres are marked by a satellite repeat and a centromere-specific retrotransposon. Plant Cell 14:1691–1704
Cheung WY, Gale MD (1990) The isolation of high molecular weight DNA from wheat, barley and rye for analysis by pulse-field gel electrophoresis. Plant Mol Biol 14:881–888
Church GM, Gilbert W (1984) Genomic sequencing. Proc Natl Acad Sci USA 81:1991–1995
Chuzhanova N, Abeysinghe SS, Krawczak M, Cooper DN (2003) Translocation and gross deletion breakpoints in human inherited disease and cancer II: potential involvement of repetitive sequence elements in secondary structure formation between DNA ends. Hum Mutat 22:245–251
Copenhaver GP, Pikaard CS (1996) Two-dimensional RFLP analyses reveal megabase-sized clusters of rRNA gene variants in Arabidopsis thaliana, suggesting local spreading of variants as the mode for gene homogenization during concerted evolution. Plant J 9:273–282
Cromie GA, Connelly JC, Leach DRF (2001) Recombination at double-strand breaks and DNA ends: conserved mechanisms from phage to humans. Mol Cell 8:1163–1174
Doležel J, Macas J, Lucretti S (1999) Flow analysis and sorting of plant chromosomes. In: Robinson JP, Darzynkiewicz Z, Dean PN, Dressler LG, Orfao A, Rabinovitch PS, Stewart CC, Tanke HJ, Wheeless LL (eds) Current protocols in cytometry. Wiley, New York, pp 5.3.1.–5.3.33
Doolittle WF, Sapienza C (1980) Selfish genes, the phenotype paradigm and genome evolution. Nature 284:601–603
Dover GA (1982) Molecular drive: a cohesive mode of species evolution. Nature 299:111–117
Dover GA (1986) Molecular drive in multigene families: how biological novelties arise, spread and are assimilated. Trends Genet 2:159–165
Driscoll CS, Sears ER (1971) Individual addition of the chromosomes of Imperial rye to wheat. Agron Abstr 1971:6
Dvorak JK, McGuire PE, Cassidy B (1988) Apparent sources of the A genome of wheat inferred from polymorphism in abundance and restriction fragment length of repeated nucleotide sequences. Genome 30:680–689
Elisaphenko EA, Nesterova TB, Duthie SM, Ruldugina OV, Rogozin IB, Brockdorff N, Zakian SM (1998) Repetitive DNA sequences in the common vole: cloning, characterization and chromosome localization of two novel complex repeats MS3 and MS4 from the genome of the East European vole Microtus rossiaemeridionalis. Chromosome Res 6:351–360
Ellis THN, Lee D, Thomas CM, Simpson PR, Cleary WG, Newman M-A, Burcham KWG (1988) 5S rRNA genes in Pisum: sequence, long range and chromosomal organization. Mol Genet Genomics 214:333–342
Flavell R (1980) The molecular characterization and organization of plant chromosomal DNA sequences. Annu Rev Plant Physiol 31:569–596
Fransz PF, Alonso-Blanco C, Liharska TB, Peeters AJM, Zabel P, de Jong JH (1996) High resolution physical mapping in Arabidopsis thaliana and tomato by fluorescence in situ hybridization to extended DNA fibre. Plant J 9:421–430
Gondo Y, Okada T, Matsuyama N, Saitoh Y, Yanagisawa Y, Ikeda J-E (1998) Human megasatellite DNA RS447: copy-number polymorphisms and interspecies conservation. Genomics 54:39–49
Gorbunova V, Levy AA (1999) How plants make ends meet: DNA double-strand break repair. Trends Plant Sci 4:263–269
Gusev VD, Nemytikova LA, Chuzhanova NA (1999) On the complexity measures of genetic sequences. Bioinformatics 15:994–999
Gustafson JP, Lukaszewski AJ, Bennett MD (1983) Somatic deletion and redistribution of telomeric heterochromatin in the genus Secale and in Triticale. Chromosoma 88:293–298
Haber JE (2000) Partners and pathways repairing a double-strand break. Trends Genet 16:259–264
Hatch FT, Mazrimas JA (1974) Fractionation and characterization of satellite DNAs of the kangaroo rat (Dipodomys ordii). Nucleic Acids Res 1:559–575
Henikoff S, Ahmad K, Malik HS (2001) The centromere paradox: stable inheritance with rapidly evolving DNA. Science 293:1098–1102
Heslop-Harrison JS, Murata M, Ogura Y, Schwarzacher T, Motoyoshi F (1999) Polymorphism and genomic organization of repetitive DNA from centromeric regions of Arabidopsis chromosomes. Plant Cell 11:31–42
Hsu H-L, Gilley D, Blackburn EH, Chen D (1999) Ku is associated with the telomere in mammals. Proc Natl Acad Sci USA 96:12454–12458
Jones JDG, Flavell R (1982) The mapping of highly repeated DNA families and their relationship to C-bands in chromosomes of Secale cereale. Chromosoma 86:595–612
de Jong JH, Zhong X-B, Fransz PF, Wennekes-van Eden J, Jacobsen E, Zabel P (2000) High resolution FISH reveals the molecular and chromosomal organization of repetitive sequences of individual tomato chromosomes. In: Olmo E, Redi CA (eds) Chromosomes Today, vol 13. Birkhauser, Switzerland, pp 267–275
Kojima KK, Kubo Y, Fujiwara H (2002) Complex and tandem repeat structure of subtelomeric regions in the Taiwan cricet, Teleogryllus taiwanemma. J Mol Evol 54:474–485
Kumekawa N, Hosouchi T, Tsuruoka H, Kotani H (2000) The size and sequence organization of the centromeric region of Arabidopsis thaliana chromosome 5. DNA Res 8:285–290
Lapitan NLV (1992) Organization and evolution of higher plant nuclear genomes. Genome 35:171–181
Lapitan NLV, Sears RG, Gill BS (1984) Translocations and other karyotypic structural changes in wheat × rye hybrids regenerated from tissue culture. Theor Appl Genet 68:547–554
Lapitan NLV, Sears RG, Rayburn AL, Gill BS (1986) Wheat-rye translocations. J Hered 77:415–419
Lee C, Wevrick R, Fisher RB, Ferguson-Smith MA, Lin CC (1997) Human centromeric DNAs. Hum Genet 100:291–304
Mao L, Devos KM, Zhu l, Gale M (1997) Cloning and genetic mapping of wheat telomere-associated sequences. Mol Genet Genomics 254:584–591
McAllister BF, Werren JH (1999) Evolution of tandemly repeated sequences: what happens at the end of an array? J Mol Biol 48:469–481
McIntyre CL, Pereira S, Moran LB, Appels R (1990) New Secale cereale (rye) DNA derivatives for the detection of rye chromosome segments in wheat. Genome 33:317–323
Muller AE, Kamisugi Y, Gruneberg R, Niedenhof I, Horold RJ, Meyer P (1999) Palindromic sequences and A+T elements promote illegitimate recombination in Nicotiana tabacum. J Mol Biol 291:29–46
Nonomura K-I, Kurata N (2001) The centromere composition of multiple repetitive sequences on rice chromosome 5. Chromosoma 110:284–291
Orgel LE, Crick FHC (1980) Selfish DNA: the ultimate parasite. Nature 284:604–607
O’Hare K, Chadwick BP, Constantinou A, Davis AJ, Mitchelson A, Tudor M (2002) A 5.9-kb tandem repeat at the euchromatin–heterochromatin boundary of the X chromosome of Drosophila melanogaster. Mol Genet Genomics 267:647–655
Panstruga R, Buschges R, Piffanelli P, Schulze-Lefert P (1998) A contiguous 60 kb genomic stretch from barley reveals molecular evidence for gene islands in a monocot genome. Nucleic Acids Res 26:1056–1062
Pelissier T, Tutois S, Tourmente S, Deragon JM, Picard G (1996) DNA regions flanking the major Arabidopsis thaliana satellite are principally enriched in Athila retroelement sequences. Genetica 97:141–151
Presting GG, Malysheva L, Fuchs J, Schubert I (1998) A Ty3/gypsy retrotransposon-like sequence localizes to the centromeric regions of cereal chromosomes. Plant J 16:721–728
Puchta H, Hohn B (1996) From centimorgans to basepairs: homologous recombination in plants. Trends Plant Sci 1:340–348
Richards EJ, Ausubel FM (1988) Isolation telomeric repeats within the Arabidopsis thaliana genome. Cell 53:127–136
Schueler MG, Higgins AW, Rudd MK, Gustashaw K, Willard HF (2001) Genomic and genetic definition of a functional human centromere. Science 294:109–115
Schwarzacher T, Heslop-Harrison JS (2000) Practical in situ hybridization. BIOS, Oxford
Siebert R, Puchta H (2002) Efficient repair of genomic double-strand breaks by homologous recombination between directly repeated sequences in the plant genome. Plant Cell 14:1121–1131
Smith GP (1976) Evolution of repeated DNA sequences by unequal crossover. Science 191:528–535
Sun X, Wahlstrom J, Karpen G (1997) Molecular structure of a functional Drosophila centromere. Cell 91:1007–1019
Svitashev SK, Pawlowski WP, Makarevitch I, Plank AW, Somers DA (2002) Complex transgene locus structures implicate multiple mechanisms for plant transgene rearrangement. Plant J 32:433–445
Sybenga J (1983) Rye chromosome nomenclature and homoeology relationships. Z Pflanzenzuchtg 90:297–304
Takata M, Sasaki MS, Sonoda E, Morrison C, Hashimoto M, Utsumi H, Yamaguchi-Iwai Y, Shinohara A, Takeda S (1998) Homologous recombination and non-homologous end-joining pathways of DNA double-strand break repair have overlapping roles in the maintenance of chromosomal integrity in vertebrate cells. EMBO J 17:5497–5508
Ugarkovic D, Plohl M (2002) Variation in satellite DNA profiles—causes and effects. EMBO J 21:5955–5959
Vergust AC, Hooykaas PJJ (1999) Recombination in the plant genome and its application in biotechnology. Crit Rev Plant Sci 18:1–31
Vershinin AV, Ellis THN (1999) Heterogeneity of the internal structure of PDR1, a family of Ty1/copia-like retrotransposons in pea. Mol Genet Genomics 262:703–713
Vershinin AV, Heslop-Harrison JS (1998) Comparative analysis of the nucleosomal structure of rye, wheat and their relatives. Plant Mol Biol 36:149–161
Vershinin AV, Schwarzacher T, Heslop-Harrison JS (1995) The large-scale organization of repetitive DNA families at the telomeres of rye chromosomes. Plant Cell 7:1823–1833
Vershinin AV, Alkhimova EG, Heslop-Harrison JS (1996) Molecular diversification of tandemly organized DNA sequences and heterochromatic chromosome regions in some Triticeae species. Chromosome Res 4:517–525
Waye JS, Willard HF (1989) Concerted evolution of alpha satellite DNA: evidence for species specificity and a general lack of sequence conservation among alphoid sequences of higher primates. Chromosoma 98:273–279
Willard HF (1989) The genomics of long tandem arrays of satellite DNA in the human genome. Genome 31:737–744
Zhong X-B, Fransz PF, Wennekes-van Eden J, Ramanna MS, van Kammen A, Zabel P, de Jong JH (1998) FISH studies reveal the molecular and chromosomal organization of individual telomere domains in tomato. Plant J 13:507–517
Zhong CX, Marshall JB, Topp C, Mroczek R, Kato A, Nagaki K, Birchler JA, Jiang J, Dawe RK (2002) Centromeric retroelements and satellites interact with maize kinetochore protein CENH3. Plant Cell 14:2825–2836
Zinic SD, Ugarkovic D, Cornudella L, Plohl M (2000) A novel interspersed type of organization of satellite DNAs in Tribolium madens heterochromatin. Chromosome Res 8:201–212
Acknowledgements
We are grateful to Dr. J. Doležel (Institute of Experimental Botany, Olomouc, Czech Republic) for providing us with sorted rye chromosomes, Dr. N. Chuzhanova (Cardiff University, Cardiff, UK) for measuring the complexity profile, and Prof. T.H.N. Ellis (John Innes Centre, Norwich, UK) for many valuable comments on the manuscript. The Institute of Cytology and Genetics is supported by the Russian Academy of Science; the Institute of Molecular Biology and Genetics is supported by the Ukraine National Academy of Sciences. This work was also supported by an INTAS grant (03-51-5908) and grants from the Russian Foundation for Basic Research (00-04-48992, 04-04-48813) and the Royal Society.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Shaw
Rights and permissions
About this article
Cite this article
Alkhimova, O.G., Mazurok, N.A., Potapova, T.A. et al. Diverse patterns of the tandem repeats organization in rye chromosomes. Chromosoma 113, 42–52 (2004). https://doi.org/10.1007/s00412-004-0294-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00412-004-0294-4