Abstract
Purpose
This study was conducted to investigate the percentages of Th22 and Th17 cells in the peripheral blood of septic patients with and without acute lung injury (ALI) and their clinical significance.
Methods
A total of 479 patients were divided into non-ALI and ALI groups. The percentages of Th22 and Th17 cells and the levels of interleukin 22 (IL-22), 6 (IL-6), and 17 (IL-17) were determined. Receiver operating characteristic curve analysis was performed to assess the diagnostic value of Th22 and Th17 cells to predict sepsis-induced ALI.
Results
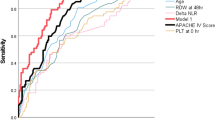
The lung injury prediction score (LIPS), IL-6, IL-17, and IL-22 levels and the percentages of Th17 and Th22 cells were significantly higher in the ALI group (P < 0.05). They were significant factors affecting sepsis-induced ALI (P < 0.05). Multivariate logistic regression analysis showed that the LIPS (OR = 1.130), IL-17 (OR = 1.982), IL-22 (OR = 2.612) and the percentages of Th17 (OR = 2.211) and Th22 (OR = 3.230) cells were independent risk factors for ALI. The area under the curve of Th22 cells was 0.844 to predict ALI with a cutoff value of 6.81%. The sensitivity and specificity for early diagnosis of sepsis-induced ALI by the Th22 cell percentage were 78.72% and 89.13%, respectively.
Conclusions
Th22 and Th17 cells in peripheral blood are significantly increased in septic patients with ALI and they may be used as biomarkers for early diagnosis of sepsis-induced ALI.


Similar content being viewed by others
Data Availability
The datasets used during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AUC:
-
Area under curve
- ALI:
-
Acute lung injury
- IL:
-
Interleukin
- ROC:
-
Receiver operating characteristic curve
- LIPS:
-
Lung injury prediction score
- TNF-α:
-
Tumor necrosis factor α
- PCT:
-
Procalcitonin
- CRP:
-
C reactive protein
- IQR:
-
Interquartile range
References
Yeh LC, Huang PW, Hsieh KH, Wang CH, Kao YK, Lin TH, Lee XL (2017) Elevated plasma levels of Gas6 are associated with acute lung injury in patients with severe sepsis. Tohoku J Exp Med 243(3):187–193. https://doi.org/10.1620/tjem.243.187
Kida Y, Ohshimo S, Shime N (2017) Optimal cutoff value for lung injury prediction score and potential confounders for identifying the risk of developing acute respiratory distress syndrome. Crit Care Med 45(6):e624–e625. https://doi.org/10.1097/CCM.0000000000002353
Butt Y, Kurdowska A, Allen TC (2016) Acute lung injury: a clinical and molecular review. Arch Pathol Lab Med 140(4):345–350. https://doi.org/10.5858/arpa.2015-0519-RA
Tang L, Bai J, Chung CS, Lomas-Neira J, Chen Y, Huang X, Ayala A (2014) Active players in resolution of shock/sepsis induced indirect lung injury: immunomodulatory effects of Tregs and PD-1. J Leukoc Biol 96(5):809–820. https://doi.org/10.1189/jlb.4MA1213-647RR
Kang MJ, Yoon CM, Nam M, Kim DH, Choi JM, Lee CG, Elias JA (2015) Role of chitinase 3-Like-1 in interleukin-18-induced pulmonary type 1, type 2, and type 17 inflammation; alveolar destruction; and airway fibrosis in the murine lung. Am J Respir Cell Mol Biol 53(6):863–871. https://doi.org/10.1165/rcmb.2014-0366OC
Raphael I, Nalawade S, Eagar TN, Forsthuber TG (2015) T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine 74(1):5–17. https://doi.org/10.1016/j.cyto.2014.09.011
Jia L, Wu C (2014) The biology and functions of Th22 cells. Adv Exp Med Biol 841:209–230. https://doi.org/10.1007/978-94-017-9487-9_8
Hernandez P, Gronke K, Diefenbach A (2018) A catch-22: interleukin-22 and cancer. Eur J Immunol 48(1):15–31. https://doi.org/10.1002/eji.201747183
Li J, Li M, Su L, Wang H, Xiao K, Deng J, Jia Y, Han G, Xie L (2015) Alterations of T helper lymphocyte subpopulations in sepsis, severe sepsis, and septic shock: a prospective observational study. Inflammation 38(3):995–1002. https://doi.org/10.1007/s10753-014-0063-3
Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP (2017) Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 43(3):304–377. https://doi.org/10.1007/s00134-017-4683-6
Seeley EJ (2013) Updates in the management of acute lung injury: a focus on the overlap between AKI and ARDS. Adv Chronic Kidney Dis 20(1):14–20. https://doi.org/10.1053/j.ackd.2012.10.001
Gajic O, Dabbagh O, Park PK, Adesanya A, Chang SY, Hou P, III Anderson H, Hoth JJ, Mikkelsen ME, Gentile NT, Gong MN, Talmor D, Bajwa E, Watkins TR, Festic E, Yilmaz M, Iscimen R, Kaufman DA, Esper AM, Sadikot R, Douglas I, Sevransky J, Malinchoc M, Illness USC, Injury Trials Group: Lung Injury Prevention Study I (2011) Early identification of patients at risk of acute lung injury: evaluation of lung injury prediction score in a multicenter cohort study. Am J Respir Crit Care Med 183(4):462–470. https://doi.org/10.1164/rccm.201004-0549OC
Shi L, Ji Q, Liu L, Shi Y, Lu Z, Ye J, Zeng T, Xue Y, Yang Z, Liu Y, Lu J, Huang X, Qin Q, Li T, Lin YZ (2020) IL-22 produced by Th22 cells aggravates atherosclerosis development in ApoE(-/-) mice by enhancing DC-induced Th17 cell proliferation. J Cell Mol Med 24(5):3064–3078. https://doi.org/10.1111/jcmm.14967
Kalbitz M, Karbach M, Braumueller S, Kellermann P, Gebhard F, Huber-Lang M, Perl M (2016) Role of complement C5 in experimental blunt chest trauma-induced septic acute lung injury (ALI). PLoS ONE 11(7):e0159417. https://doi.org/10.1371/journal.pone.0159417
Szabo K, Papp G, Szanto A, Tarr T, Zeher M (2016) A comprehensive investigation on the distribution of circulating follicular T helper cells and B cell subsets in primary Sjogren's syndrome and systemic lupus erythematosus. Clin Exp Immunol 183(1):76–89. https://doi.org/10.1111/cei.12703
Lu T, Liu Y, Yu S, Yin C, Li P, Ye J, Ma D, Ji C (2016) Increased frequency of circulating Th22 cells in patients with B cell non-Hodgkin's lymphoma. Oncotarget 7(35):56574–56583. https://doi.org/10.18632/oncotarget.10966
Azizi G, Simhag A, El Rouby NM, Mirshafiey A (2015) Th22 cells contribution in immunopathogenesis of rheumatic diseases. Iran J Allergy Asthma Immunol 14(3):246–254
Liu T, Peng L, Yu P, Zhao Y, Shi Y, Mao X, Chen W, Cheng P, Wang T, Chen N, Zhang J, Liu X, Li N, Guo G, Tong W, Zhuang Y, Zou Q (2012) Increased circulating Th22 and Th17 cells are associated with tumor progression and patient survival in human gastric cancer. J Clin Immunol 32(6):1332–1339. https://doi.org/10.1007/s10875-012-9718-8
de Lima SE, de Sousa JR, de Sousa Aarao TL, Fuzii HT, Dias Junior LB, Carneiro FR, Quaresma JA (2015) New immunologic pathways in the pathogenesis of leprosy: role for Th22 cytokines in the polar forms of the disease. J Am Acad Dermatol 72(4):729–730. https://doi.org/10.1016/j.jaad.2014.11.023
Liu X, Yang J, Deng W (2017) The inflammatory cytokine IL-22 promotes murine gliomas via proliferation. Exp Ther Med 13(3):1087–1092. https://doi.org/10.3892/etm.2017.4059
Gupta DL, Bhoi S, Mohan T, Galwnkar S, Rao DN (2016) Coexistence of Th1/Th2 and Th17/Treg imbalances in patients with post traumatic sepsis. Cytokine 88:214–221. https://doi.org/10.1016/j.cyto.2016.09.010
Kumar V (2018) T cells and their immunometabolism: a novel way to understanding sepsis immunopathogenesis and future therapeutics. Eur J Cell Biol 97(6):379–392. https://doi.org/10.1016/j.ejcb.2018.05.001
Chen J, Liu H, Li L, Wang H, Li Y, Wang Y, Ding K, Hao S, Shao Y, Li L, Song J, Wang G, Shao Z, Fu R (2019) Abnormal numbers of CD4+ T lymphocytes and abnormal expression of CD4+ T lymphocytesecreted cytokines in patients with immunerelated haemocytopenia. Mol Med Rep 20(5):3979–3990. https://doi.org/10.3892/mmr.2019.10663
Zheng R, Wang F, Huang Y, Xiang Q, Dai H, Zhang W (2020) Elevated Th17 cell frequencies and Th17/Treg ratio are associated with airway hyperresponsiveness in asthmatic children. J Asthma. https://doi.org/10.1080/02770903.2020.1737710
McAleer JP, Kolls JK (2014) Directing traffic: IL-17 and IL-22 coordinate pulmonary immune defense. Immunol Rev 260(1):129–144. https://doi.org/10.1111/imr.12183
Jie Z, Liang Y, Yi P, Tang H, Soong L, Cong Y, Zhang K, Sun J (2017) Retinoic acid regulates immune responses by promoting IL-22 and modulating S100 proteins in viral hepatitis. J Immunol 198(9):3448–3460. https://doi.org/10.4049/jimmunol.1601891
Zhang Y, Cobleigh MA, Lian JQ, Huang CX, Booth CJ, Bai XF, Robek MD (2011) A proinflammatory role for interleukin-22 in the immune response to hepatitis B virus. Gastroenterology 141(5):1897–1906. https://doi.org/10.1053/j.gastro.2011.06.051
Liang SC, Nickerson-Nutter C, Pittman DD, Carrier Y, Goodwin DG, Shields KM, Lambert AJ, Schelling SH, Medley QG, Ma HL, Collins M, Dunussi-Joannopoulos K, Fouser LA (2010) IL-22 induces an acute-phase response. J Immunol 185(9):5531–5538. https://doi.org/10.4049/jimmunol.0904091
Young RS, Wiles BM, McGee DW (2017) IL-22 enhances TNF-alpha- and IL-1-induced CXCL8 responses by intestinal epithelial cell lines. Inflammation 40(5):1726–1734. https://doi.org/10.1007/s10753-017-0614-5
Mizoguchi A, Yano A, Himuro H, Ezaki Y, Sadanaga T, Mizoguchi E (2018) Clinical importance of IL-22 cascade in IBD. J Gastroenterol 53(4):465–474. https://doi.org/10.1007/s00535-017-1401-7
Jiang S, Shan F, Zhang Y, Jiang L, Cheng Z (2018) Increased serum IL-17 and decreased serum IL-10 and IL-35 levels correlate with the progression of COPD. Int J Chron Obstruct Pulmon Dis 13:2483–2494. https://doi.org/10.2147/COPD.S167192
Guan X, Lu Y, Wang G, Gibson P, Chen F, Fang K, Wang Z, Pang Z, Guo Y, Lu J, Yuan Y, Ran N, Wang F (2018) The role of regulatory T cell in nontypeable haemophilus influenzae-induced acute exacerbation of chronic obstructive pulmonary disease. Mediat Inflamm 2018:8387150. https://doi.org/10.1155/2018/8387150
Funding
This study was supported by Medical Health Science and Technology Projects, Zhejiang Provincial Health Commission, Zhejiang, China (Grant Nos. 2016–33 and 2020KY441).
Author information
Authors and Affiliations
Contributions
GL and LZ designed the study. LZ, NH and KZ collected the data and performed analysis. GL, LZ and HL drafted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics Approval
This study was approved by the ethical committee of Zhejiang Provincial People's Hospital.
Informed Consent
Written informed consent was obtained from every patient.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, G., Zhang, L., Han, N. et al. Increased Th17 and Th22 Cell Percentages Predict Acute Lung Injury in Patients with Sepsis. Lung 198, 687–693 (2020). https://doi.org/10.1007/s00408-020-00362-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-020-00362-1