Abstract
Introduction
Th17 cells play a crucial role in neutrophilic inflammation and tissue injury in non-cystic fibrosis (non-CF) bronchiectasis. Clarithromycin demonstrates anti-inflammatory and immunomodulatory properties but the effect of long-term clarithromycin prophylaxis on the Th17 response in non-CF bronchiectasis is still unexplored.
Methods
Th17 response was studied in 22 patients with stable non-CF bronchiectasis receiving daily 500-mg clarithromycin for 12 weeks. We analysed IL-17 concentrations in exhaled breath condensate (EBC) and peripheral blood Th17 cells, whereas functional parameters and clinical data were recorded in parallel.
Results
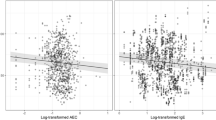
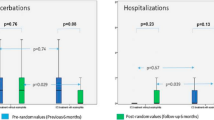
Both, post-treatment absolute counts of CD4+IL17+ cells in peripheral blood and IL-17 levels in EBC decreased significantly (post-treatment CD4+IL17+ mean 2.418 ± 0.414 cells/μl versus pre-treatment 3.202 ± 0.507 cells/μl, p = 0.036 and post-treatment IL-17 mean levels 7.16 ± 0.47 pg/ml versus pre-treatment 9.32 ± 0.47 pg/ml, p < 0.001, respectively). Post-treatment EBC IL-17 levels decreased significantly in both patients who exhibited exacerbations and those who remained stable during the study period (mean 6.72 ± 0.37 versus 9.12 ± 0.64 pg/ml, p = 0.01 and 7.69 ± 0.9 versus 9.53 ± 0.72 pg/ml, p = 0.042, respectively), while pre-treatment and post-treatment levels did not differ between the two groups (p = 0.665 and p = 0.465, respectively). PaO2 improved significantly (post-treatment mean 77.73 ± 2.23 mmHg versus pre-treatment 73.18 ± 2.22 mmHg, p = 0.025), while PaCO2, post-bronchodilation FEV1, and post-bronchodilation FVC remained unaltered.
Conclusions
Our results argue for a reduction of both systemic and local Th17 response after prophylactic, low-dose clarithromycin administration in patients with non-CF bronchiectasis, suggestive of a potential anti-inflammatory and/or immunomodulatory action.



Similar content being viewed by others
References
Cole PJ (1986) Inflammation: a two-edged sword–the model of bronchiectasis. Eur J Respir Dis Suppl 147:6–15
Barker AF (2002) Bronchiectasis. N Engl J Med 346(18):1383–1393
Aujla SJ, Dubin PJ, Kolls JK (2007) Interleukin-17 in pulmonary host defense. Exp Lung Res 33(10):507–518
Iwakura Y, Nakae S, Saijo S, Ishigame H (2008) The roles of IL-17A in inflammatory immune responses and host defense against pathogens. Immunol Rev 226:57–79
Bettelli E, Korn T, Oukka M, Kuchroo VK (2008) Induction and effector functions of Th 17 cells. Nature 453(7198):1051–1057
Steinman L (2007) A brief history of Th 17, the first major revision in the Th 1/Th 2 hypothesis of T cell-mediated tissue damage. Nat Med 13(2):139–145
Vanaudenaerde BM, Verleden SE, Vos R et al (2011) Innate and adaptive interleukin-17-producing lymphocytes in chronic inflammatory lung disorders. Am J Respir Crit Care Med 183(8):977–986
Dubin PJ, McAllister F, Kolls JK (2007) Is cystic fibrosis a TH17 disease? Inflamm Res 56(6):221–227
Kolios G, Manousou P, Bourikas L et al (2006) Ciprofloxacin inhibits cytokine-induced nitric oxide production in human colonic epithelium. Eur J Clin Invest 36(10):720–729
Rubin BK, Henke MO (2004) Immunomodulatory activity and effectiveness of macrolides in chronic airway disease. Chest 125(2 Suppl):70S–78S
Shinkai M, Tamaoki J, Kobayashi H et al (2006) Clarithromycin delays progression of bronchial epithelial cells from G1 phase to S phase and delays cell growth via extracellular signal-regulated protein kinase suppression. Antimicrob Agents Chemother 50(5):1738–1744
Tsang WK, Bilton D (2009) Clinical challenges in managing bronchiectasis. Respirology 14(5):637–650
Banerjee D, Honeybourne D, Khair OA (2004) The effect of oral clarithromycin on bronchial airway inflammation in moderate-to-severe stable COPD: a randomized controlled trial. Treat Respir Med 3(1):59–65
Horváth I, Hunt J, Barnes PJ et al (2005) ATS/ERS task force on exhaled breath condensate. exhaled breath condensate: methodological recommendations and unresolved questions. Eur Respir J 26(3):523–548
Miller MR, Crapo R, Hankinson J et al (2005) ATS/ERS task force. General considerations for lung function testing. Eur Respir J 26(1):153–161
Sun YC, Zhou QT, Yao WZ (2005) Sputum interleukin-17 is increased and associated with airway neutrophilia in patients with severe asthma. Chin Med J (Engl) 118(11):953–956
Di Stefano A, Caramori G, Gnemmi I et al (2009) T helper type 17-related cytokine expression is increased in the bronchial mucosa of stable chronic obstructive pulmonary disease patients. Clin Exp Immunol 157:316–324
McAllister F, Henry A, Kreindler JL et al (2005) Role of IL-17A, IL-17F, and the IL-17 receptor in regulating growth-related oncogene-alpha and granulocyte colony-stimulating factor in bronchial epithelium: implications for airway inflammation in cystic fibrosis. J Immunol 175(1):404–412
Tan HL, Regamey N, Brown S et al (2011) The Th17 pathway in cystic fibrosis lung disease. Am J Respir Crit Care Med 184(2):252–258
Ding FM, Zhu SL, Shen C et al (2012) Low-dose clarithromycin therapy modulates CD4(+) T-cell responses in a mouse model of chronic Pseudomonas aeruginosa lung infection. Respirology 17(4):727–734
Tiringer K, Treis A, Fucik P et al (2013) A Th17- and Th2-skewed cytokine profile in cystic fibrosis lungs represents a potential risk factor for Pseudomonas aeruginosa infection. Am J Respir Crit Care Med 187(6):621–629
Wong C, Jayaram L, Karalus N et al (2012) Azithromycin for prevention of exacerbations in non-cystic fibrosis bronchiectasis (EMBRACE): a randomised, double-blind, placebo-controlled trial. Lancet 380(9842):660–667
Altenburg J, de Graaff CS, Stienstra Y et al (2013) Effect of azithromycin maintenance treatment on infectious exacerbations among patients with non-cystic fibrosis bronchiectasis: the BAT randomized controlled trial. JAMA 309(12):1251–1259
Kushwah R, Gagnon S, Sweezey NB (2013) Intrinsic predisposition of naïve cystic fibrosis T cells to differentiate towards a Th17 phenotype. Respir Res 14:138
Ishimatsu Y, Kadota J, Iwashita T et al (2004) Macrolide antibiotics induce apoptosis of human peripheral lymphocytes in vitro. Int J Antimicrob Agents 24(3):247–253
Kadota J, Mizunoe S, Kishi K et al (2005) Antibiotic-induced apoptosis in human activated peripheral lymphocytes. Int J Antimicrob Agents 25(3):216–220
Loukides S, Kontogianni K, Hillas G et al (2011) Exhaled breath condensate in asthma: from bench to bedside. Curr Med Chem 18(10):1432–1443
Borrill ZL, Roy K, Singh D (2008) Exhaled breath condensate biomarkers in COPD. Eur Respir J 32(2):472–486
Robroeks CM, Roozeboom MH, de Jong PA et al (2010) Structural lung changes, lung function, and non-invasive inflammatory markers in cystic fibrosis. Pediatr Allergy Immunol 21(3):493–500
Karakoc GB, Inal A, Yilmaz M et al (2009) Exhaled breath condensate MMP-9 levels in children with bronchiectasis. Pediatr Pulmonol 44(10):1010–1016
Zihlif N, Paraskakis E, Tripoli C et al (2006) Markers of airway inflammation in primary ciliary dyskinesia studied using exhaled breath condensate. Pediatr Pulmonol 41(6):509–514
Brodlie M, McKean MC, Johnson GE et al (2011) Raised interleukin-17 is immunolocalised to neutrophils in cystic fibrosis lung disease. Eur Respir J 37(6):1378–1385
Eustace A, Smyth LJ, Mitchell L et al (2011) Identification of cells expressing IL-17A and IL-17F in the lungs of patients with COPD. Chest 139(5):1089–1100
Loukides S, Bouros D, Papatheodorou G et al (2002) Exhaled H2O2 in steady-state bronchiectasis: relationship with cellular composition in induced sputum, spirometry, and extent and severity of disease. Chest 121(1):81–88
Tsang KW, Ho PI, Chan KN et al (1999) A pilot study of low-dose erythromycin in bronchiectasis. Eur Respir J 13(2):361–364
Feldman C, Anderson R, Theron AJ et al (1997) Roxithromycin, clarithromycin, and azithromycin attenuate the injurious effects of bioactive phospholipids on human respiratory epithelium in vitro. Inflammation 21(6):655–665
Pukhalsky AL, Shmarina GV, Kapranov NI et al (2004) Anti-inflammatory and immunomodulating effects of clarithromycin in patients with cystic fibrosis lung disease. Mediat Inflamm 13(2):111–117
Yalçin E, Kiper N, Ozçelik U et al (2006) Effects of claritromycin on inflammatory parameters and clinical conditions in children with bronchiectasis. J Clin Pharm Ther 31(1):49–55
Altenburg J, de Graaff CS, van der Werf TS et al (2011) Immunomodulatory effects of macrolide antibiotics—part 2: advantages and disadvantages of long-term, low-dose macrolide therapy. Respiration 81(1):75–87
Acknowledgments
Dr. Fouka has received grant support over the past 3 years from the Hellenic Thoracic Society to perform research on the immunomodulating effects of clarithromycin on patients with bronchiectasis using exhaled breath condensate. All authors also confirm that the manuscript is not under consideration by any other publication.
Conflict of interest
All authors accept direct responsibility for the manuscript and declare no financial or other relative conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fouka, E., Lamprianidou, E., Arvanitidis, K. et al. Low-Dose Clarithromycin Therapy Modulates Th17 Response In Non-Cystic Fibrosis Bronchiectasis Patients. Lung 192, 849–855 (2014). https://doi.org/10.1007/s00408-014-9619-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-014-9619-0