Abstract
Background
Although emphysema destroys alveolar structures progressively and causes death eventually, no drug has been discovered to prevent, intervene, and/or resolve this life-threatening disease. We recently reported that sulfated caffeic acid dehydropolymer CDSO3 is a novel potent triple-action inhibitor of elastolysis, oxidation, and inflammation in vitro, and therefore, a potential anti-emphysema agent. However, the in vivo therapeutic potency, duration and mode of actions, and effective route remain to be demonstrated.
Methods
Emphysema was induced in rats with human sputum elastase (HSE) combined with cigarette smoke extract (CSE). CDSO3 at 5, 30, or 100 μg/kg was dosed to the lung or injected subcutaneously at 2, 6, or 24 h before or 1 or 24 h or 1 week after the HSE/CSE instillation. At 1 h or 48 h or on day 21–22 or day 28, lungs were examined for airway-to-blood injurious barrier damage; their elastolytic, oxidative, and inflammatory activities; lung luminal leukocytes infiltration; functional treadmill exercise endurance; and/or morphological airspace enlargement.
Results
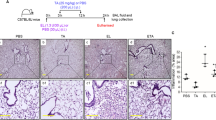
CDSO3, when dosed to the lung at 30 or 100 μg/kg, but not via systemic subcutaneous injection, significantly (43–93 %) attenuated HSE/CSE-induced (1) barrier damage measured by luminal hemorrhage and protein leak; (2) elastolytic, oxidative, and inflammatory activities measured with elastase, reduced glutathione, and TNFα levels, respectively; (3) luminal neutrophil infiltration and tissue myeloperoxidase activity; (4) functional impairment of exercise endurance; and (5) airspace enlargement, in both preventive and interventional dosing protocols. Notably, the effects were shown to last for 24 h at the greater 100-μg/kg dose, and the 1-week-delayed administration was also capable of attenuating the development of emphysema.
Conclusions
CDSO3 is a novel, potent, long-acting, nonpeptidic macromolecule that inhibits HSE/CSE-induced elastolysis, oxidation, and inflammation in the lung and thereby attenuates the development of emphysema in rats, in both preventive and interventional manners, when administered locally to the lung.




Similar content being viewed by others
Abbreviations
- AAALAC:
-
Association for assessment and accreditation of laboratory animal care
- ANOVA:
-
Analysis of variance
- BAL:
-
Bronchoalveolar lavage
- BALF:
-
BAL fluid
- BCA:
-
Bicinchoninic assay
- βNADPH:
-
β-Nicotinamide adenine dinucleotide 2′-phosphate
- CA:
-
Caffeic acid
- CDSO3:
-
Sulfated dehydropolymer of caffeic acid
- COPD:
-
Chronic obstructive pulmonary disease
- CSE:
-
Cigarette smoke extract
- DTNB:
-
5,5′-Dithiobis-(2-nitrobenzoic acid)
- EDTA:
-
Ethylenediaminetetraacetic acid
- GSSG:
-
Glutathione disulfide
- HEPES:
-
(2-Hydroxyethyl)piperazine-N′-2-ethanesulfonic acid
- HSE:
-
Human sputum elastase
- HTAB:
-
Hexadecyltrimethyl ammonium bromide
- rGSH:
-
Reduced glutathione
- MLI:
-
Mean linear intercept
- MPO:
-
Myeloperoxidase
- tGSH:
-
Total glutathione
- SD:
-
Standard deviation
- SE:
-
Standard error
- TNFα:
-
Tumor necrosis factor α
- 2-VP:
-
2-Vinyl pyridine
References
Kemp SV, Polkey MI, Shah PL (2009) The epidemiology, etiology, clinical features, and natural history of emphysema. Thorac Surg Clin 19:149–158
Tuder RM, Petrache I (2012) Pathogenesis of chronic obstructive pulmonary disease. J Clin Invest 122:2749–2755
Tuder RM, Voelkel NF (2008) Pathobiology of emphysema. In: Voelkel NF, MacNee W (eds) Chronic obstructive lung diseases, vol 2. BC Decker, Hamilton, Ontario, Canada, pp 63–75
Barnes PJ, Stockley RA (2005) COPD: current therapeutic interventions and future approaches. Eur Respir J 25:1084–1106
Wise RA, Tashkin DP (2007) Optimizing treatment of chronic obstructive pulmonary disease: an assessment of current therapy. Am J Med 120:S4–S13
American Lung Association (2013) Chronic obstructive pulmonary disease (COPD) fact sheet. Available at http://www.lung.org/lung-disease/copd/resources/facts-figures/COPD-Fact-Sheet.html
Fischer BM, Pavlisko E, Voynow JA (2011) Pathogenic triad in COPD: oxidative stress, protease-antiprotease imbalance, and inflammation. Int J Chron Obstruct Pulmon Dis 6:413–421
Gardi C, Arezzini B, Martorana PA (2008) Testing of compounds in models of pulmonary emphysema. Curr Med Chem 115:803–808
Wright JL, Cosio M, Churg A (2008) Animal models of chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol 295:L1–L15
Saluja B, Thakkar JN, Li H, Desai UR, Sakagami M (2013) Novel low molecular weight lignins as potential anti-emphysema agents: in vitro triple inhibitory activity against elastase, oxidation and inflammation. Pulm Pharmacol Ther 26:296–304
Monien BH, Henry BL, Raghuraman A, Hindle M, Desai UR (2006) Novel chemo-enzymatic oligomers of cinnamic acids as direct and indirect inhibitors of coagulation proteinases. Bioorg Med Chem 14:7988–7998
Henry BL, Monien BH, Bock PE, Desai UR (2007) A novel allosteric pathway of thrombin inhibition: exosite II mediated potent inhibition of thrombin by chemo-enzymatic, sulfated dehydropolymers of 4-hydroxycinnamic acids. J Biol Chem 282:31891–31899
Rahman I, Kode A, Biswas SK (2006) Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc 1:3159–3165
Shinguh Y, Yamazaki A, Inamura N, Fujie K, Okamoto M, Nakahara K, Notsu Y, Okuhara M, Ono T (1998) Biochemical and pharmacological characterization of FR134043, a novel elastase inhibitor. Eur J Pharmacol 345:299–308
Goldblum SE, Wu K-M, Jay M (1985) Lung myeloperoxidase as a measure of pulmonary leukostasis in rabbits. J Apply Physiol 59:1978–1985
Thurlbeck WM (1967) Internal surface area and other measurements in emphysema. Thorax 22:483–496
Kuraki T, Ishibashi M, Takayama M, Shiraishi M, Yoshida M (2002) A novel oral neutrophil elastase inhibitor (ONO-6818) inhibits human neutrophil elastase-induced emphysema in rats. Am J Repir Crit Care Med 166:496–500
Fujie K, Shinguh Y, Yamazaki A, Hatanaka M, Okamoto M, Okuhara (1999) Inhibition of elastase-induced acute inflammation and pulmonary emphysema in hamsters by a novel neutrophil elastase inhibitor FR901277. Inflamm Res 48:160–167
Williams JC, Falcome RC, Knee C, Stein RL, Strimpler AM, Reaves B, Giles RE, Krell RD (1991) Biologic characterization of ICI 200,880 and ICI 200,355, novel inhibitors of human neutrophil elastase. Am Rev Respir Dis 144:875–883
Ohbayashi H (2002) Neutrophil elastase inhibitors as treatment for COPD. Expert Opin Investig Drugs 11:965–980
Suzuki M, Betsuyaku T, Ito Y, Nagai K, Edajima M, Moriyama C, Nasuhara Y, Nishimura M (2009) Curcumin attenuates elastase- and cigarette smoke-induced pulmonary emphysema in mice. Am J Physiol Lung Cell Mol Physiol 296:L614–L623
Demura Y, Taraseviciene-Stewart L, Scerbavicius R, Tuder RM, Voelkel NF (2004) N-acetylcysteine treatment protects against VEGF-receptor blockade-related emphysema. COPD 1:25–32
Biswas S, Hwang JW, Kirkham PA, Rahman I (2013) Pharmacological and dietary antioxidant therapies for chronic obstructive pulmonary disease. Curr Med Chem 20:1496–1530
Birrell MA, Wong S, Hele DJ, McCluskie K, Hardaker E, Belvisi MG (2005) Steroid-resistant inflammation in a rat model of chronic obstructive pulmonary disease is associated with a lack of nuclear factor-kappaB pathway activation. Am J Respir Crit Care Med 172:74–84
Tanaka K, Sato K, Aoshiba K, Azuma A, Mizushima T (2012) Superiority of PC-SOD to other anti-COPD drugs for elastase-induced emphysema and alteration in lung mechanics and respiratory function in mice. Am J Physiol Lung Cell Mol Physiol 302:L1250–L1261
Lee J, Lee D, Kim E, Choe K, Oh Y, Shim T, Kim S, Lee Y, Lee S (2005) Simvastatin inhibits cigarette smoking-induced emphysema and pulmonary hypertension in rat lungs. Am J Respire Crit Care Med 172:987–993
Sakagami M (2010) Targeted drug delivery through the respiratory system: molecular control on lung absorption and disposition. In: Smyth HDC, Hickey AJ (eds) Controlled pulmonary drug delivery. Springer, New York, pp 127–141
Spencer JL, Stone PJ, Nugent MA (2006) New insights into the inhibition of human neutrophil elastase by heparin. Biochemistry 45:9104–9120
Rao NV, Kennedy TP, Rao G, Ky N, Hoidal JR (1990) Sulfated polysaccharides prevent human leukocyte elastase-induced acute lung injury and emphysema in hamsters. Am Rev Respir Dis 142:407–412
Qi Y, Zhao G, Liu D, Shriver Z, Sundaram M, Sengupta S, Venkataraman G, Langer R, Sasisekharan R (2004) Delivery of therapeutic levels of heparin and low-molecular-weight heparin through a pulmonary route. Proc Natl Acad Sci USA 101:9867–9872
Lapenna D, Mezzetti A, de Gioia S, Ciofani G, Marzio L, Di Ilio C, Cuccurullo F (1992) Heparin: does it act as an antioxidant in vivo? Biochem Pharmacol 44:188–191
Stone PJ, Lucey EC, Calore JD, McMahon MP, Snider GL, Franz-blau C (1988) Defenses of the hamster lung against human neutrophil and porcine pancreatic elastase. Respiration 54:1–15
Acknowledgments
This research was funded by an American Lung Association Biomedical Research Grant (MS; RG-159601-N), VCU Presidential Research Quest Fund (MS), Medical College of Virginia Foundation (BS and MS), and National Institutes of Health (URD; HL090586 and HL107152). BS acknowledges the financial support from the VCU School of Pharmacy and the VCU School of Graduate Studies Dissertation Award for her graduate studies.
Conflict of interest
The authors do not have financial or nonfinancial competing interests regarding this article. BS, URD, and MS are the inventors of a US patent titled “Cinnamic acid-based oligomers and uses thereof” (8,491,872), parts of which are included in this article.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Saluja, B., Li, H., Desai, U.R. et al. Sulfated Caffeic Acid Dehydropolymer Attenuates Elastase and Cigarette Smoke Extract–induced Emphysema in Rats: Sustained Activity and a Need of Pulmonary Delivery. Lung 192, 481–492 (2014). https://doi.org/10.1007/s00408-014-9597-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-014-9597-2