Abstract
Background
Expression of transforming growth factor (TGF)-β1 and increases in angiogenesis and deposition of extracellular matrix are the key features of tracheal granulation formation. The aim of this study was to investigate the potential role of thalidomide in preventing granulation tissue formation from the aspect of cellular effects in vitro, including fibroblast proliferation, vascular endothelial growth factor (VEGF) release, and collagen production.
Methods
Human lung fibroblasts were obtained from bronchus and cultured. The effects of thalidomide on cell proliferation, migration, TGF-β1-induced VEGF, and signal pathway were investigated.
Results
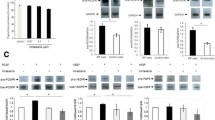
Thalidomide (20 μM) not only inhibited cell proliferation after 24 h [fold increase of cell number, 0.85 ± 0.09 vs. 1.47 ± 0.14 (treatment vs. control group); P < 0.01] and 48 h of incubation (0.85 ± 0.10 vs. 1.97 ± 0.12; P < 0.001), it also inhibited cell migration and slowed wound closure at 24 h (P < 0.001). Thalidomide significantly attenuated TGF-β1-induced VEGF expression at both the mRNA and protein levels. Incubation of thalidomide with cells stimulated with TGF-β1 significantly inhibited their production of collagen. Thalidomide inhibited Smad3, STAT3, and subsequent p44/42 kinase phosphorylation.
Conclusion
Thalidomide may inhibit human fibroblast proliferation and it is worthy of further in vivo investigation.






Similar content being viewed by others
References
Law JH, Barnhart K, Rowlett W, de la Rocha O, Lowenberg S (1993) Increased frequency of obstructive airway abnormalities with long-term tracheostomy. Chest 104(1):136–138
Liu H, Chen JC, Holinger LD, Gonzalez-Crussi F (1995) Histopathologic fundamentals of acquired laryngeal stenosis. Pediatr Pathol Lab Med 15(5):655–677
Karagiannidis C, Velehorschi V, Obertrifter B, Macha HN, Linder A, Freitag L (2006) High-level expression of matrix-associated transforming growth factor-beta1 in benign airway stenosis. Chest 129(5):1298–1304
Pokharel RP, Maeda K, Yamamoto T, Noguchi K, Iwai Y, Nakamura H, Iijima K (1999) Expression of vascular endothelial growth factor in exuberant tracheal granulation tissue in children. J Pathol 188(1):82–86
Lee YC, Hung MH, Liu LY, Chang KT, Chou TY, Wang YC, Wu YC, Lai CL, Tsai CC, Su KC, Perng DW (2011) The roles of transforming growth factor-beta and vascular endothelial growth factor in the tracheal granulation formation. Pulm Pharmacol Ther 24(1):23–31
Galluccio G, Lucantoni G, Battistoni P, Paone G, Batzella S, Lucifora V, Dello Iacono R (2009) Interventional endoscopy in the management of benign tracheal stenoses: definitive treatment at long-term follow-up. Eur J Cardiothorac Surg 35(3):429–433
Ciccone AM, De Giacomo T, Venuta F, Ibrahim M, Diso D, Coloni GF, Rendina EA (2004) Operative and non-operative treatment of benign subglottic laryngotracheal stenosis. Eur J Cardiothorac Surg 26(4):818–822
Grillo HC, Donahue DM, Mathisen DJ, Wain JC, Wright CD (1995) Postintubation tracheal stenosis. Treatment and results. J Thorac Cardiovasc Surg 109(3):486–492 discussion 492–483
Rea F, Callegaro D, Loy M, Zuin A, Narne S, Gobbi T, Grapeggia M, Sartori F (2002) Benign tracheal and laryngotracheal stenosis: surgical treatment and results. Eur J Cardiothorac Surg 22(3):352–356
Ward RF, April MM (1998) Mitomycin-C in the treatment of tracheal cicatrix after tracheal reconstruction. Int J Pediatr Otorhinolaryngol 44(3):221–226
Shlomi D, Peled N, Shitrit D, Bendayan D, Amital A, Kramer MR (2008) Protective effect of immunosuppression on granulation tissue formation in metallic airway stents. Laryngoscope 118(8):1383–1388
Keifer JA, Guttridge DC, Ashburner BP, Baldwin AS Jr (2001) Inhibition of NF-kappa B activity by thalidomide through suppression of IkappaB kinase activity. J Biol Chem 276(25):22382–22387
Corral LG, Haslett PA, Muller GW, Chen R, Wong LM, Ocampo CJ, Patterson RT, Stirling DI, Kaplan G (1999) Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-alpha. J Immunol 163(1):380–386
Or R, Feferman R, Shoshan S (1998) Thalidomide reduces vascular density in granulation tissue of subcutaneously implanted polyvinyl alcohol sponges in guinea pigs. Exp Hematol 26(3):217–221
Bartlett JB, Dredge K, Dalgleish AG (2004) The evolution of thalidomide and its IMiD derivatives as anticancer agents. Nat Rev Cancer 4(4):314–322
Strauss RM, Bate J, Nischal KK, Clayton T, Gooi J, Darling JC, Newton-Bishop JA (2006) A child with laryngo-onychocutaneous syndrome partially responsive to treatment with thalidomide. Br J Dermatol 155(6):1283–1286
Moreira AL, Friedlander DR, Shif B, Kaplan G, Zagzag D (1999) Thalidomide and a thalidomide analogue inhibit endothelial cell proliferation in vitro. J Neurooncol 43(2):109–114
Miyata M, Tamura E, Motoki K, Nagata K, Yamazoe Y (2003) Thalidomide-induced suppression of embryo fibroblast proliferation requires CYP1A1-mediated activation. Drug Metab Dispos 31(4):469–475
Fuchida SI, Shimazaki C, Hirai H, Akamatsu S, Yamada N, Uchida R, Okano A, Okamoto M, Inaba T, Taniwaki M (2008) The effects of thalidomide on chemotactic migration of multiple myeloma cell lines. Int J Lab Hematol 30(3):220–229
Komorowski J, Jerczynska H, Siejka A, Baranska P, Lawnicka H, Pawlowska Z, Stepien H (2006) Effect of thalidomide affecting VEGF secretion, cell migration, adhesion and capillary tube formation of human endothelial EA.hy 926 cells. Life Sci 78(22):2558–2563
Ashcroft GS, Yang X, Glick AB, Weinstein M, Letterio JL, Mizel DE, Anzano M, Greenwell-Wild T, Wahl SM, Deng C, Roberts AB (1999) Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat Cell Biol 1(5):260–266
Verrecchia F, Chu ML, Mauviel A (2001) Identification of novel TGF-beta/Smad gene targets in dermal fibroblasts using a combined cDNA microarray/promoter transactivation approach. J Biol Chem 276(20):17058–17062
Blobe GC, Schiemann WP, Lodish HF (2000) Role of transforming growth factor beta in human disease. N Engl J Med 342(18):1350–1358
Leask A, Abraham DJ (2004) TGF-beta signaling and the fibrotic response. FASEB J 18(7):816–827
Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D, Heller R, Ellis LM, Karras J, Bromberg J, Pardoll D, Jove R, Yu H (2002) Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene 21(13):2000–2008
Bartoli M, Platt D, Lemtalsi T, Gu X, Brooks SE, Marrero MB, Caldwell RB (2003) VEGF differentially activates STAT3 in microvascular endothelial cells. FASEB J 17(11):1562–1564
Bartoli M, Gu X, Tsai NT, Venema RC, Brooks SE, Marrero MB, Caldwell RB (2000) Vascular endothelial growth factor activates STAT proteins in aortic endothelial cells. J Biol Chem 275(43):33189–33192
Acknowledgments
We thank all the staff members who participated in the study, especially our chief laboratory technician, Mrs. Mo-Ci Wu. This work was supported by research grants from Taipei Veterans General Hospital (V99C1-100).
Conflict of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
C.-M. Tseng and Y.-H. Hsiao contributed equally to this work.
Rights and permissions
About this article
Cite this article
Tseng, CM., Hsiao, YH., Su, V.YF. et al. The Suppression Effects of Thalidomide on Human Lung Fibroblasts: Cell Proliferation, Vascular Endothelial Growth Factor Release, and Collagen Production. Lung 191, 361–368 (2013). https://doi.org/10.1007/s00408-013-9477-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-013-9477-1