Abstract
Background
Moderate normobaric hyperoxia causes alveolar and vascular lung derangement in the newborn rat. Endogenous nitric oxide (NO), which promotes lung growth, is produced from the metabolism of l-arginine to l-citrulline in endothelial cells. We investigated whether administering l-citrulline by raising the serum levels of l-arginine and enhancing NO endogenous synthesis attenuates moderate hyperoxia-induced lung injury.
Methods
Newborn rats were exposed to FiO2 = 0.6 or room air for 14 days to induce lung derangement and then were administered l-citrulline or a vehicle (sham). Lung histopathology was studied with morphometric features. Lung tissues and bronchoalveolar lavage fluid (BALF) were collected for analysis. Lung vascular endothelial growth factor (VEGF), nitric oxide synthase (eNOS), and matrix metalloproteinase 2 (MMP2) gene and protein expressions were assessed.
Results
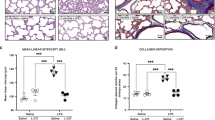
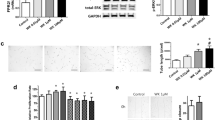
Serum l-arginine rose in the L-citr + hyperoxia group (p = 0.05), as well as the Von Willebrand factor stained vessels count (p = 0.0008). Lung VEGF immune staining, localized on endothelial cells, was weaker in the sections under hyperoxia than the l-citr + hyperoxia and room air groups. This pattern was comparable with the VEGF gene and protein expression profiles. Mean alveolar size increased in the untreated hyperoxia and sham-treated groups compared with the groups reared in room air or treated with l-citrulline under exposure to hyperoxia (p = 0.0001). Lung VEGF and eNOS increased in the l-citrulline-treated rats, though this treatment did not change MMP2 gene expression but regulated the MMP2 active protein, which rose in BALF (p = 0.003).
Conclusions
We conclude that administering l-citrulline proved effective in improving alveolar and vascular growth in a model of oxygen-induced pulmonary damage, suggesting better lung growth and matrix regulation than in untreated groups.




Similar content being viewed by others
Abbreviations
- BPD:
-
Bronchopulmonary dysplasia
- MMP:
-
Matrix metalloproteinase
- BALF:
-
Bronchoalveolar lavage fluid
- l-citr:
-
l-Citrulline
References
Filippone M, Sartor M, Zacchello F, Baraldi E (2003) Flow limitation in infants with bronchopulmonary dysplasia and respiratory function at school age. Lancet 361:753–754
Balasubramaniam V, Mervis CF, Maxey AM, Markham NE, Abman SH (2007) Hyperoxia reduces bone marrow, circulating, and lung endothelial progenitor cells in the developing lung: implications for the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 292:L1073–L1084
Balasubramaniam V, Ingram DA (2009) Endothelial progenitors in the risk of developing bronchopulmonary dysplasia: can we include endothelial progenitor cells in BPD risk assessment? Am J Respir Crit Care Med 180:488–490
Abman SH, Baker C, Balasubramaniam V (2008) Growth and development of the lung circulation: mechanisms and clinical implications. In: Bancalari E (ed) The newborn lung. Saunders Elsevier, Philadelphia, pp 50–72
Jakkula M, Le Cras TD, Gebb S, Hirth KP, Tuder RM, Voelkel NF, Abman SH (2000) Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am J Physiol Lung Cell Mol Physiol 279:L600–L607
Tang K, Rossiter HB, Wagner PD, Breen EC (2004) Lung-targeted VEGF inactivation leads to an emphysema phenotype in mice. J Appl Physiol 97:1559–1566
Kallapur SG, Bachurski CJ, Le Cras TD, Joshi SN, Ikegami M, Jobe AH (2004) Vascular changes after intra-amniotic endotoxin in preterm lamb lungs. Am J Physiol Lung Cell Mol Physiol 287:L1178–L1185
Lin YJ, Markham NE, Balasubramaniam V, Tang JR, Maxey A, Kinsella JP, Abman SH (2005) Inhaled nitric oxide enhances distal lung growth after exposure to hyperoxia in neonatal rats. Pediatr Res 58:22–29
McCurnin DC, Pierce RA, Chang LY, Gibson LL, Osborne-Lawrence S, Yoder BA, Kerecman JD, Albertine KH, Winter VT, Coalson JJ, Crapo JD, Grubb PH, Shaul PW (2005) Inhaled NO improves early pulmonary function and modifies lung growth and elastin deposition in a baboon model of neonatal chronic lung disease. Am J Physiol Lung Cell Mol Physiol 288:L450–L459
ter Horst SA, Walther FJ, Poorthuis BJ, Hiemstra PS, Wagenaar GT (2007) Inhaled nitric oxide attenuates pulmonary inflammation and fibrin deposition and prolongs survival in neonatal hyperoxic lung injury. Am J Physiol Lung Cell Mol Physiol 293:L35–L44
Bode-Böger SM, Muke J, Surdacki A, Brabant G, Böger RH, Frölich JC (2003) Oral 1-arginine improves endothelial function in healthy individuals older than 70 years. Vasc Med 8:77–81
Piatti PM, Monti LD, Valsecchi G, Magni F, Setola E, Marchesi F, Galli-Kienle M, Pozza G, Alberti KG (2001) Long-term oral l-arginine administration improves peripheral and hepatic insulin sensitivity in type 2 diabetic patients. Diabetes Care 24:875–880
Walker HA, McGing E, Fisher I, Böger RH, Bode-Böger SM, Jackson G, Ritter JM, Chowienczyk PJ (2001) Endothelium-dependent vasodilation is independent of the plasma l-arginine/ADMA ratio in men with stable angina: lack of effect of oral l-arginine on endothelial function, oxidative stress and exercise performance. J Am Coll Cardiol 38:499–505
Morris SM Jr (2004) Enzymes of arginine metabolism. J Nutr 134:2743S–2747S
Curis E, Nicolis I, Moinard C, Osowska S, Zerrouk N, Benazeth S, Cynober L (2005) Almost all about l-citrulline in mammals. Amino Acids 29:177–205
Waugh WH, Daeschner CW, Files BA, McConnell ME, Strandjord SE (2001) Oral l-citrulline as arginine precursor may be beneficial in sickle cell disease: early phase two results. J Natl Med Assoc 93:363–371
Schwedhelm E, Maas R, Freese R, Jung D, Lukacs Z, Jambrecina A, Spickler W, Schulze F, Böger RH (2008) Pharmacokinetic and pharmacodynamic properties of oral l-citrulline and l-arginine: impact on nitric oxide metabolism. Br J Clin Pharmacol 65:51–59
Solomonson LP, Flam BR, Pendleton LC, Goodwin BL, Eichler DC (2003) The caveolar nitric oxide synthase/arginine regeneration system for NO production in endothelial cells. J Exp Biol 206:2083–2087
Greenlee KJ, Werb Z, Kheradmand F (2007) Matrix metalloproteinases in lung: multiple, multifarious, and multifaceted. Physiol Rev 87:69–98
Chetty C, Lakka SS, Bhoopathi P, Rao JS (2010) MMP-2 alters VEGF expression via alphaVbeta3 integrin-mediated PI3 K/AKT signaling in A549 lung cancer cells. Int J Cancer 127:1081–1095
Guidolin D, Albertin G, Spinazzi R, Sorato E, Mascarin A, Cavallo D, Antonello M, Ribatti D (2008) Adrenomedullin stimulates angiogenic response in cultured human vascular endothelial cells: involvement of the vascular endothelial growth factor receptor 2. Peptides 29:2013–2023
Kubista M, Andrade JM, Bengtsson M, Forootan A, Jonak J, Lind K, Sindelka R, Sjoback R, Sjogreen B, Strombom L, Stahlberg A, Zoric N (2006) The real-time polymerase chain reaction. Mol Aspects Med 27:95–125
Onisto M, Slongo ML, Gregnanin L, Gastaldi T, Carli M, Rosolen A (2005) Expression and activity of vascular endothelial growth factor and metalloproteinases in alveolar and embryonal rhabdomyosarcoma cell lines. Int J Oncol 27:791–798
Thurlbeck WM (1975) Postnatal growth and development of the lung. Am Rev Respir Dis 111:803–844
Yi M, Jankov RP, Belcastro R, Humes D, Copland I, Shek S, Sweezey NB, Post M, Albertine KH, Auten RL, Tanswell AK (2004) Opposing effects of 60% oxygen and neutrophil influx on alveologenesis in the neonatal rat. Am J Respir Crit Care Med 170:1188–1196
Masood A, Yi M, Lau M, Belcastro R, Shek S, Pan J, Kantores C, McNamara PJ, Kavanagh BP, Belik J, Jankov RP, Tanswell AK (2009) Therapeutic effects of hypercapnia on chronic lung injury and vascular remodeling in neonatal rats. Am J Physiol Lung Cell Mol Physiol 297:L920–L930
Donohue PK, Gilmore MM, Cristofalo E, Wilson RF, Weiner JZ, Lau BD, Robinson KA, Allen MC (2011) Inhaled nitric oxide in preterm infants: a systematic review. Pediatrics 127:e414–e422
Vadivel A, Aschner JL, Rey-Parra GJ, Magarik J, Zeng H, Summar M, Eaton F, Thébaud B (2010) L-citrulline attenuates arrested alveolar growth and pulmonary hypertension in oxygen-induced lung injury in newborn rats. Pediatr Res 68:519–525
Husain AN, Siddiqui NH, Stocker JT (1998) Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum Pathol 29:710–717
Jobe AH, Bancalari E (2001) Bronchopulmonary dysplasia. Am J Respir Crit Care Med 163:1723–1729
Duda DG, Fukumura D, Jain RK (2004) Role of eNOS in neovascularization: NO for endothelial progenitor cells. Trends Mol Med 10:143–145
Ziche M, Morbidelli L, Choudhuri R, Zhang HT, Donnini S, Granger HJ, Bicknell R (1997) Nitric oxide synthase lies downstream from vascular endothelial growth factor-induced but not basic fibroblast growth factor-induced angiogenesis. J Clin Invest 99:2625–2634
Dulak J, Józkowicz A (2003) Regulation of vascular endothelial growth factor synthesis by nitric oxide: facts and controversies. Antioxid Redox Signal 5:123–132
Ananthakrishnan M, Barr FE, Summar ML, Smith HA, Kaplowitz M, Cunningham G, Magarik J, Zhang Y, Fike CD (2009) L-citrulline ameliorates chronic hypoxia-induced pulmonary hypertension in newborn piglets. Am J Physiol Lung Cell Mol Physiol 297:L506–L511
Crosby LM, Waters CM (2010) Epithelial repair mechanisms in the lung. Am J Physiol Lung Cell Mol Physiol 298:L715–L731
van Hinsbergh VW, Engelse MA, Quax PH (2006) Pericellular proteases in angiogenesis and vasculogenesis. Arterioscler Thromb Vasc Biol 26:716–728
Walter A, Etienne-Selloum N, Sarr M, Kane MO, Beretz A, Schini-Kerth VB (2008) Angiotensin II induces the vascular expression of VEGF and MMP-2 in vivo: preventive effect of red wine polyphenols. J Vasc Res 45:386–394
Le NT, Xue M, Castelnoble LA, Jackson CJ (2007) The dual personalities of matrix metalloproteinases in inflammation. Front Biosci 12:1475–1487
Kheradmand F, Rishi K, Werb Z (2002) Signaling through the EGF receptor controls lung morphogenesis in part by regulating MT1-MMP-mediated activation of gelatinase A/MMP2. J Cell Sci 115:839–848
Danan C, Jarreau PH, Franco ML, Dassieu G, Grillon C, Abd Alsamad I, Lafuma C, Harf A, Delacourt C (2002) Gelatinase activities in the airways of premature infants and development of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 283:L1086–L1093
Schulz CG, Sawicki G, Lemke RP, Roeten BM, Schulz R, Cheung PY (2004) MMP-2 and MMP-9 and their tissue inhibitors in the plasma of preterm and term neonates. Pediatr Res 55:794–801
Acknowledgments
The authors are indebted to Professor Franco Zacchello, who strongly encouraged the creation of the Neonatal Developmental Biology Group, and to Professor A. S. Belloni for her kind advice. The authors also thank the personnel at the Department of Anatomy and Physiology (G. Sarasin, A. Rambaldo, and D. Guidolin), at the Pathology Section of the Department of Oncological and Surgical Sciences, and at the NICU, and the Associazione Pulcino. This work was funded by the Italian Ministry of Health grant CPDA081132/08, University of Padova, Padova, Italy.
Conflicts of interest
The authors have no conflict of interests or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
D. Grisafi and E. Tassone contributed equally to this work.
Rights and permissions
About this article
Cite this article
Grisafi, D., Tassone, E., Dedja, A. et al. L-citrulline Prevents Alveolar and Vascular Derangement in a Rat Model of Moderate Hyperoxia-induced Lung Injury. Lung 190, 419–430 (2012). https://doi.org/10.1007/s00408-012-9382-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-012-9382-z