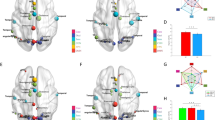
Abstract
Hubs in the brain network are the regions with high centrality and are crucial in the network communication and information integration. Patients with schizophrenia (SCZ) exhibit wide range of abnormality in the hub regions and their connected functional connectivity (FC) at the whole-brain network level. Study of the hubs in the brain networks supporting complex social behavior (social brain network, SBN) would contribute to understand the social dysfunction in patients with SCZ. Forty-nine patients with SCZ and 27 healthy controls (HC) were recruited to undertake the resting-state magnetic resonance imaging scanning and completed a social network (SN) questionnaire. The resting-state SBN was constructed based on the automatic analysis results from the NeuroSynth. Our results showed that the left temporal lobe was the only hub of SBN, and its connected FCs strength was higher than the remaining FCs in both two groups. SCZ patients showed the lower association between the hub-connected FCs (compared to the FCs not connected to the hub regions) with the real-life SN characteristics. These results were replicated in another independent sample (30 SCZ and 28 HC). These preliminary findings suggested that the hub-connected FCs of SBN in SCZ patients exhibit the abnormality in predicting real-life SN characteristics.




Similar content being viewed by others
References
Fornito A, Zalesky A, Breakspear M (2015) The connectomics of brain disorders. Nat Rev Neurosci 16:159–172. https://doi.org/10.1038/nrn3901
van den Heuvel MP, Sporns O (2011) Rich-club organization of the human connectome. J Neurosci 31:15775–15786. https://doi.org/10.1523/JNEUROSCI.3539-11.2011
Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews-Hanna JR, Sperling RA, Johnson KA (2009) Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to alzheimer’s disease. J Neurosci 29:1860–1873. https://doi.org/10.1523/JNEUROSCI.5062-08.2009
He Y, Wang J, Wang L, Chen ZJ, Yan C, Yang H, Tang H, Zhu C, Gong Q, Zang Y, Evans AC (2009) Uncovering intrinsic modular organization of spontaneous brain activity in humans. PLoS ONE 4:e5226. https://doi.org/10.1371/journal.pone.0005226
van den Heuvel MP, Sporns O, Collin G, Scheewe T, Mandl RC, Cahn W, Goni J, Hulshoff Pol HE, Kahn RS (2013) Abnormal rich club organization and functional brain dynamics in schizophrenia. JAMA Psychiat 70:783–792. https://doi.org/10.1001/jamapsychiatry.2013.1328
Zalesky A, Fornito A, Bullmore ET (2010) Network-based statistic: identifying differences in brain networks. Neuroimage 53:1197–1207. https://doi.org/10.1016/j.neuroimage.2010.06.041
Howes OD, Murray RM (2014) Schizophrenia: an integrated sociodevelopmental-cognitive model. Lancet (London, England) 383:1677–1687. https://doi.org/10.1016/S0140-6736(13)62036-X
Porcelli S, Van Der Wee N, van der Werff S, Aghajani M, Glennon JC, van Heukelum S, Mogavero F, Lobo A, Olivera FJ, Lobo E, Posadas M, Dukart J, Kozak R, Arce E, Ikram A, Vorstman J, Bilderbeck A, Saris I, Kas MJ, Serretti A (2019) Social brain, social dysfunction and social withdrawal. Neurosci Biobehav Rev 97:10–33. https://doi.org/10.1016/j.neubiorev.2018.09.012
Crossley N (2015) Social network analysis for ego-nets. Sage Publications Ltd, Thousand Oaks. https://doi.org/10.4135/9781473911871
Rubinov M, Bullmore E (2013) Schizophrenia and abnormal brain network hubs. Dialogues Clin Neurosci 15:339–349. https://doi.org/10.31887/DCNS.2013.15.3/mrubinov
Collin G, Kahn RS, de Reus MA, Cahn W, van den Heuvel MP (2014) Impaired rich club connectivity in unaffected siblings of schizophrenia patients. Schizophr Bull 40:438–448. https://doi.org/10.1093/schbul/sbt162
Mier D, Kirsch P (2015) Social-cognitive deficits in schizophrenia. Curr Top Behav Neurosci. https://doi.org/10.1007/7854_2015_427
Adolphs R (2009) The social brain: neural basis of social knowledge. Annu Rev Psychol 60:693–716. https://doi.org/10.1146/annurev.psych.60.110707.163514
Kennedy DP, Adolphs R (2012) The social brain in psychiatric and neurological disorders. Trends Cogn Sci 16:559–572. https://doi.org/10.1016/j.tics.2012.09.006
Burns JK (2004) An evolutionary theory of schizophrenia: Cortical connectivity, metarepresentation, and the social brain. Behav Brain Sci 27:831–855. https://doi.org/10.1017/S0140525X04000196 ((discussion 855–885))
Burns J (2006) The social brain hypothesis of schizophrenia. World Psychiatry 5:77–81
Bickart KC, Dickerson BC, Barrett LF (2014) The amygdala as a hub in brain networks that support social life. Neuropsychologia 63:235–248. https://doi.org/10.1016/j.neuropsychologia.2014.08.013
Dasgupta S, Tyler SC, Wicks J, Srinivasan R, Grossman ED (2017) Network connectivity of the right STS in three social perception localizers. J Cogn Neurosci 29:221–234. https://doi.org/10.1162/jocn_a_01054
Levy J, Goldstein A, Zagoory-Sharon O, Weisman O, Schneiderman I, Eidelman-Rothman M, Feldman R (2016) Oxytocin selectively modulates brain response to stimuli probing social synchrony. Neuroimage 124:923–930. https://doi.org/10.1016/j.neuroimage.2015.09.066
Lahnakoski JM, Glerean E, Salmi J, Jääskeläinen IP, Sams M, Hari R, Nummenmaa L (2012) Naturalistic FMRI mapping reveals superior temporal sulcus as the hub for the distributed brain network for social perception. Front Hum Neurosci 6:233. https://doi.org/10.3389/fnhum.2012.00233
Amodio DM, Frith CD (2006) Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci 7:268–277. https://doi.org/10.1038/nrn1884
Cohen S, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM Jr (1997) Social ties and susceptibility to the common cold. JAMA 277:1940–1944. https://doi.org/10.1001/jama.1997.03540480040036
Morgan C, Bhugra D (2010) Principles of social psychiatry. Princ Soc Psychiatry. https://doi.org/10.1002/9780470684214
Noonan MP, Mars RB, Sallet J, Dunbar RIM, Fellows LK (2018) The structural and functional brain networks that support human social networks. Behav Brain Res 355:12–23. https://doi.org/10.1016/j.bbr.2018.02.019
Bickart KC, Wright CI, Dautoff RJ, Dickerson BC, Barrett LF (2011) Amygdala volume and social network size in humans. Nat Neurosci 14:163–164. https://doi.org/10.1038/nn.2724
Kanai R, Bahrami B, Roylance R, Rees G (2012) Online social network size is reflected in human brain structure. Proc Biol Sci 279:1327. https://doi.org/10.1098/rspb.2011.1959
Von DHR, Vyas G, Olson IR (2014) The social network-network: Size is predicted by brain structure and function in the amygdala and paralimbic regions. Soc Cogn Affect Neurosci 9:1962–1972. https://doi.org/10.1093/scan/nsu009
Dziura SL, Thompson JC (2014) Social-network complexity in humans is associated with the neural response to social information. Psychol Sci 25:2095–2101. https://doi.org/10.1177/0956797614549209
Jasper C (2013) Amygdalae enlargement and activation are associated with social network complexity in individuals with human immunodeficiency virus (HIV). J Undergrad Rev 9:68–74
Preller KH, Herdener M, Schilbach L, Stämpfli P, Hulka LM, Vonmoos M, Ingold N, Vogeley K, Tobler PN, Seifritz E (2014) Functional changes of the reward system underlie blunted response to social gaze in cocaine users. Proc Natl Acad Sci USA 111:2842–2847. https://doi.org/10.1073/pnas.1317090111
Bickart KC, Hollenbeck MC, Barrett LF, Dickerson BC (2012) Intrinsic amygdala-cortical functional connectivity predicts social network size in humans. J Neurosci 32:14729. https://doi.org/10.1523/JNEUROSCI.1599-12.2012
Zou LQ, Yang ZY, Yi W, Lui SSY, Chen AT, Cheung EFC, Chan RCK (2016) What does the nose know? Olfactory function predicts social network size in human. Sci Rep 6:25026. https://doi.org/10.1038/srep25026
Zhang YJ, Pu CC, Wang YM, Zhang RT, Cai XL, Zhou SZ, Ma YT, Wang Y, Cheung EFC, Lui SSY, Yu X, Chan RCK (2021) Social brain network correlates with real-life social network in individuals with schizophrenia and social anhedonia. Schizophr Res 232:77–84. https://doi.org/10.1016/j.schres.2021.05.016
Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD (2011) Large-scale automated synthesis of human functional neuroimaging data. Nat Methods 8:665–670. https://doi.org/10.1038/nmeth.1635
First MB, Gibbon M, Spitzer RL, Williams JB (1996) User’s guide for the structured clinical interview for DSM-IV axis I disorders research version. Biometrics Research Department, New York State Psychiatric Institute, New York. https://doi.org/10.1037/t07827-000
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders, 5th edn. American Psychiatric Association, Washington, DC. https://doi.org/10.1176/appi.books.9780890425596
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC (1998) The mini-international neuropsychiatric interview (mINI): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59(Suppl 20):22–33 ((quiz 34–57))
Li L, Bachevalier J, Hu X, Klin A, Preuss TM, Shultz S, Jones W (2018) Topology of the structural social brain network in typical adults. Brain Connect 8:537–548. https://doi.org/10.1089/brain.2018.0592
Rubinov M, Sporns O (2010) Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52:1059–1069. https://doi.org/10.1016/j.neuroimage.2009.10.003
Dai Z, Yan C, Li K, Wang Z, Wang J, Cao M, Lin Q, Shu N, Xia M, Bi Y, He Y (2015) Identifying and mapping connectivity patterns of brain network hubs in Alzheimer’s disease. Cereb Cortex 25:3723–3742. https://doi.org/10.1093/cercor/bhu246
Wang L, Metzak PD, Honer WG, Woodward TS (2010) Impaired efficiency of functional networks underlying episodic memory-for-context in schizophrenia. J Neurosci 30:13171–13179. https://doi.org/10.1523/JNEUROSCI.3514-10.2010
Wang J, Wang X, Xia M, Liao X, Evans A, He Y (2015) Gretna: a graph theoretical network analysis toolbox for imaging connectomics. Front Hum Neurosci 9:386. https://doi.org/10.3389/fnhum.2015.00458
Kim M, Kim D, Bae S, Han DH, Jeong B (2020) Aberrant structural network of comorbid attention deficit/hyperactivity disorder is associated with addiction severity in internet gaming disorder. Neuroimage Clin 27:102263. https://doi.org/10.1016/j.nicl.2020.102263
Yoo K, Rosenberg MD, Hsu WT, Zhang S, Li CR, Scheinost D, Constable RT, Chun MM (2018) Connectome-based predictive modeling of attention: comparing different functional connectivity features and prediction methods across datasets. Neuroimage 167:11–22. https://doi.org/10.1016/j.neuroimage.2017.11.010
Crossley NA, Mechelli A, Scott J, Carletti F, Fox PT, McGuire P, Bullmore ET (2014) The hubs of the human connectome are generally implicated in the anatomy of brain disorders. Brain 137:2382–2395. https://doi.org/10.1093/brain/awu132
Kirsch HE (2006) Social cognition and epilepsy surgery. Epilepsy Behav 8:71–80. https://doi.org/10.1016/j.yebeh.2005.09.002
Alonso-Vanegas MA, Cisneros-Franco JM, Castillo-Montoya C, Martínez-Rosas AR, Gómez-Pérez ME, Rubio-Donnadieu F (2013) Self-reported quality of life in pharmacoresistant temporal lobe epilepsy: correlation with clinical variables and memory evaluation. Epileptic Disord 15:263–271. https://doi.org/10.1684/epd.2013.0590
Guo JY, Huhtaniska S, Miettunen J, Jääskeläinen E, Kiviniemi V, Nikkinen J, Moilanen J, Haapea M, Mäki P, Jones PB, Veijola J, Isohanni M, Murray GK (2015) Longitudinal regional brain volume loss in schizophrenia: relationship to antipsychotic medication and change in social function. Schizophr Res 168:297–304. https://doi.org/10.1016/j.schres.2015.06.016
Acknowledgements
This study was supported by grants from the Beijing Municipal Science and Technology Commission Grant (Z161100000216138), National Key Research and Development Programme (2016YFC0906402), Beijing Training Project for Leading Talents in S&T (Z151100000315020), and the CAS Key Laboratory of Mental Health, Institute of Psychology. All authors declare that the research was conducted in the absence of any commercial or financial relationship that could be constructed as a potential conflict of interests.
Author information
Authors and Affiliations
Contributions
YJZ designed the study, collected the data in the replication sample, analyzed and interpreted the data, and wrote up the drafts of the manuscript. YL collected the main sample data, interpreted the findings and commented the draft critically. ZYY, YMW, SKW helped to collect the main sample data and interpreted the findings. CCP, SZZ, YTM helped to collect the replication sample data and interpreted the findings. YW, SSYL and XY interpreted the findings and commented the drafts critically. RCKC generated the idea, designed the study, interpreted the findings, and commented the draft critically.
Corresponding author
Ethics declarations
Conflict of interest
None to declare.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, Yj., Li, Y., Wang, Ym. et al. Hub-connected functional connectivity within social brain network weakens the association with real-life social network in schizophrenia patients. Eur Arch Psychiatry Clin Neurosci 272, 1033–1043 (2022). https://doi.org/10.1007/s00406-021-01344-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-021-01344-x