Abstract
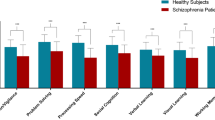
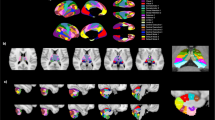
Studies have indicated thalamus-related network dysfunction in schizophrenia and psychotic disorders. However, whether thalamus-related functional connectivity (FC) contributes to the psychopathology and cognitive deficits of early-stage schizophrenia requires further investigation. A total of 34 patients with early-stage schizophrenia (illness duration = 1.62 ± 1.16 years; age = 26.00 ± 6.34 years) and 34 age- and sex-matched healthy controls were enrolled in our study and underwent comprehensive assessments of the clinical symptoms of schizophrenia, working memory tasks, and resting-state FC magnetic resonance imaging. The patients with early-stage schizophrenia had increased FC of the thalamus with the bilateral postcentral and temporal gyri, inferior occipital cortex, and temporal pole and decreased FC of the thalamus with the vestibulocerebellum and frontal pole compared with the controls. Furthermore, increased FC between the thalamus and temporal pole was positively correlated with positive scores of the Positive and Negative Syndrome Scale for Schizophrenia (PANSS) and negatively correlated with performance on working memory tasks in early-stage schizophrenia. Increased FC of the thalamus with the inferior occipital cortex was positively associated with negative PANSS scores and negatively correlated with Personal and Social Performance Scale scores in early-stage schizophrenia. Our results supported the vital role of thalamus-related network dysfunction in the psychopathology and cognitive deficits of early-stage schizophrenia.


Similar content being viewed by others
References
McGrath J, Saha S, Chant D, Welham J (2008) Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev 30:67–76
Chong HY, Teoh SL, Wu DB, Kotirum S, Chiou CF, Chaiyakunapruk N (2016) Global economic burden of schizophrenia: a systematic review. Neuropsychiatr Dis Treat 12:357–373
Siever LJ, Davis KL (2004) The pathophysiology of schizophrenia disorders: perspectives from the spectrum. Am J Psychiatry 161:398–413
Byne W, Hazlett EA, Buchsbaum MS, Kemether E (2009) The thalamus and schizophrenia: current status of research. Acta Neuropathol 117:347–368
Wagner G, De la Cruz F, Schachtzabel C, Gullmar D, Schultz CC, Schlosser RG, Bar KJ, Koch K (2015) Structural and functional dysconnectivity of the fronto-thalamic system in schizophrenia: a DCM-DTI study. Cortex 66:35–45
Andreasen NC, O’Leary DS, Cizadlo T, Arndt S, Rezai K, Ponto LL, Watkins GL, Hichwa RD (1996) Schizophrenia and cognitive dysmetria: a positron-emission tomography study of dysfunctional prefrontal-thalamic-cerebellar circuitry. Proc Natl Acad Sci USA 93:9985–9990
Ferri J, Ford JM, Roach BJ, Turner JA, van Erp TG, Voyvodic J, Preda A, Belger A, Bustillo J, O’Leary D, Mueller BA, Lim KO, McEwen SC, Calhoun VD, Diaz M, Glover G, Greve D, Wible CG, Vaidya JG, Potkin SG, Mathalon DH. Resting-state thalamic dysconnectivity in schizophrenia and relationships with symptoms. Psychol Med. 2018:1–8
Woodward ND, Heckers S (2016) Mapping thalamocortical functional connectivity in chronic and early stages of psychotic disorders. Biol Psychiatry 79:1016–1025
Williams LE, Avery SN, Woolard AA, Heckers S (2012) Intact relational memory and normal hippocampal structure in the early stage of psychosis. Biol Psychiatry 71:105–113
Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL (2008) Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol 100:3328–3342
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage 15:273–289
Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC (1995) Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med 33:636–647
Lee SH, Niznikiewicz M, Asami T, Otsuka T, Salisbury DF, Shenton ME, McCarley RW (2016) Initial and progressive gray matter abnormalities in insular gyrus and temporal pole in first-episode schizophrenia contrasted with first-episode affective psychosis. Schizophr Bull 42:790–801
Xu L, Qin W, Zhuo C, Zhu J, Liu H, Liu X, Xu Y, Yu C (2015) Selective functional disconnection of the dorsal subregion of the temporal pole in schizophrenia. Sci Rep 5:11258
Kasai K, Shenton ME, Salisbury DF, Onitsuka T, Toner SK, Yurgelun-Todd D, Kikinis R, Jolesz FA, McCarley RW (2003) Differences and similarities in insular and temporal pole MRI gray matter volume abnormalities in first-episode schizophrenia and affective psychosis. Arch Gen Psychiatry 60:1069–1077
Crespo-Facorro B, Nopoulos PC, Chemerinski E, Kim JJ, Andreasen NC, Magnotta V (2004) Temporal pole morphology and psychopathology in males with schizophrenia. Psychiatry Res 132:107–115
Bigelow J, Ng CW, Poremba A (2016) Local field potential correlates of auditory working memory in primate dorsal temporal pole. Brain Res 1640:299–313
Sasabayashi D, Takayanagi Y, Takahashi T, Koike S, Yamasue H, Katagiri N, Sakuma A, Obara C, Nakamura M, Furuichi A, Kido M, Nishikawa Y, Noguchi K, Matsumoto K, Mizuno M, Kasai K, Suzuki M (2017) Increased occipital gyrification and development of psychotic disorders in individuals with an at-risk mental state: a multicenter Study. Biol Psychiatry 82:737–745
Brewer WJ, Francey SM, Wood SJ, Jackson HJ, Pantelis C, Phillips LJ, Yung AR, Anderson VA, McGorry PD (2005) Memory impairments identified in people at ultra-high risk for psychosis who later develop first-episode psychosis. Am J Psychiatry 162:71–78
Picard H, Amado I, Mouchet-Mages S, Olie JP, Krebs MO (2008) The role of the cerebellum in schizophrenia: an update of clinical, cognitive, and functional evidences. Schizophr Bull 34:155–172
Andreasen NC, Nopoulos P, O’Leary DS, Miller DD, Wassink T, Flaum M (1999) Defining the phenotype of schizophrenia: cognitive dysmetria and its neural mechanisms. Biol Psychiatry 46:908–920
Varambally S, Venkatasubramanian G, Thirthalli J, Janakiramaiah N, Gangadhar BN (2006) Cerebellar and other neurological soft signs in antipsychotic-naive schizophrenia. Acta Psychiatr Scand 114:352–356
Rasser PE, Schall U, Peck G, Cohen M, Johnston P, Khoo K, Carr VJ, Ward PB, Thompson PM (2010) Cerebellar grey matter deficits in first-episode schizophrenia mapped using cortical pattern matching. Neuroimage 53:1175–1180
Du Y, Fryer SL, Fu Z, Lin D, Sui J, Chen J, Damaraju E, Mennigen E, Stuart B, Loewy RL, Mathalon DH, Calhoun VD (2018) Dynamic functional connectivity impairments in early schizophrenia and clinical high-risk for psychosis. NeuroImage 180:632–645
Haijma SV, Van Haren N, Cahn W, Koolschijn PC, Hulshoff Pol HE, Kahn RS (2013) Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophrenia Bull 39:1129–1138
Crespo-Facorro B, Roiz-Santianez R, Pelayo-Teran JM, Rodriguez-Sanchez JM, Perez-Iglesias R, Gonzalez-Blanch C, Tordesillas-Gutierrez D, Gonzalez-Mandly A, Diez C, Magnotta VA, Andreasen NC, Vazquez-Barquero JL (2007) Reduced thalamic volume in first-episode non-affective psychosis: correlations with clinical variables, symptomatology and cognitive functioning. NeuroImage 35:1613–1623
Acknowledgements
We thank Mr I-Fan Hu for his friendship and support. We thank Dr MHC, Dr JWH and Dr PCT, who designed the study and wrote the protocol and manuscripts, Dr TPS, Dr CTL, Dr SJT, Dr KLH, Dr YMB, and Dr WCL, who assisted with the preparation and proofreading of the manuscript, and Dr PCT and Miss WCC, who provided advice on MRI analysis and statistical analysis.
Funding
The study was supported by a grant from Taipei Veterans General Hospital (V106B-020, V107B-010, V107C-181) and Ministry of Science and Technology, Taiwan (107-2314-B-075-063-MY3). The funding source had no role in any process of our study. All authors have no financial relationships relevant to this article to disclose.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
No conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, MH., Chang, WC., Bai, YM. et al. Cortico-thalamic dysconnection in early-stage schizophrenia: a functional connectivity magnetic resonance imaging study. Eur Arch Psychiatry Clin Neurosci 270, 351–358 (2020). https://doi.org/10.1007/s00406-019-01003-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-019-01003-2