Abstract
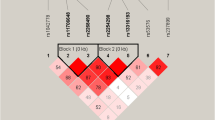
The psychosis phenotype is expressed across a continuum known as schizotypy, which ranges from personality variation through subclinical symptoms to severe psychopathology. The study of subclinical manifestations in non-affected individuals minimizes confounding factors associated with the clinical phenotype and facilitates the differentiation of dimension-specific etiological mechanisms. The aim of the present study was to investigate the association between the variation in the regulator of G-protein signaling 4 (RGS4) gene, a putative candidate gene for psychosis previously associated with schizophrenia endophenotypes, and psychotic-like experiences (PLEs). In total, 808 healthy individuals completed the community assessment of psychic experiences (CAPE) to measure positive and negative PLEs and provided a DNA sample. Two RGS4 single-nucleotide polymorphisms (SNPs) (rs951436 [SNP4] and rs2661319 [SNP18]) were genotyped. Analyses of covariance (ANCOVA) were used to explore the association of positive and negative PLEs with RGS4 variation. Our results showed associations of positive and negative PLEs with the two polymorphisms studied: subjects with the T allele (SNP4) and the A allele (SNP18) had higher scores on both the positive and the negative dimensions. Haplotypic analyses supported these results, showing the highest scores in those with the TA haplotype (SNP4-SNP18). The RGS4 variants might exert gene-specific modulating effects on psychosis proneness.

Similar content being viewed by others
References
Berman DM, Wilkie TM, Gilman AG (1996) GAIP and RGS4 are GTPase-activating proteins for the Gi subfamily of G protein alpha subunits. Cell 86:445–452
Paspalas CD, Selemon LD, Arnsten AF (2009) Mapping the regulator of G protein signaling 4 (RGS4): presynaptic and postsynaptic substrates for neuroregulation in prefrontal cortex. Cereb Cortex 19:2145–2155. doi:10.1093/cercor/bhn235
Mirnics K, Middleton FA, Stanwood GD et al (2001) Disease-specific changes in regulator of G-protein signaling 4 (RGS4) expression in schizophrenia. Mol Psychiatry 6:293–301. doi:10.1038/sj.mp.4000866
Gurling HMD, Kalsi G, Brynjolfson J et al (2001) Genomewide genetic linkage analysis confirms the presence of susceptibility loci for schizophrenia, on chromosomes 1q32.2, 5q33.2, and 8p21-22 and provides support for linkage to schizophrenia, on chromosomes 11q23.3-24 and 20q12.1-11.23. Am J Hum Genet 68:661–673. doi:10.1086/318788
Brzustowicz LM, Hayter JE, Hodgkinson KA et al (2002) Fine mapping of the schizophrenia susceptibility locus on chromosome 1q22. Hum Hered 54:199–209. doi:10.1159/000070665
Rosa A, Fañanás L, Cuesta MJMJ et al (2002) 1q21-q22 Locus is associated with susceptibility to the reality-distortion syndrome of schizophrenia spectrum disorders. Am J Med Genet 114:516–518. doi:10.1002/ajmg.10526
Williams NM, Preece A, Spurlock G et al (2004) Support for RGS4 as a susceptibility gene for schizophrenia. Biol Psychiatry 55:192–195. doi:10.1016/j.biopsych.2003.11.002
Chowdari KV, Mirnics K, Semwal P et al (2002) Association and linkage analyses of RGS4 polymorphisms in schizophrenia. Hum Mol Genet 11:1373–1380
So H-C, Chen RYL, Chen EYH et al (2008) An association study of RGS4 polymorphisms with clinical phenotypes of schizophrenia in a Chinese population. Am J Med Genet Part B 147B:77–85. doi:10.1002/ajmg.b.30577
Vilella E, Costas J, Sanjuan J et al (2008) Association of schizophrenia with DTNBP1 but not with DAO, DAOA, NRG1 and RGS4 nor their genetic interaction. J Psychiatr Res 42:278–288. doi:10.1016/j.jpsychires.2007.02.005
Ishiguro H, Horiuchi Y, Koga M et al (2007) RGS4 is not a susceptibility gene for schizophrenia in Japanese: association study in a large case-control population. Schizophr Res 89:161–164. doi:10.1016/j.schres.2006.09.015
Talkowski ME, Seltman H, Bassett AS et al (2006) Evaluation of a susceptibility gene for schizophrenia: genotype based meta-analysis of RGS4 polymorphisms from thirteen independent samples. Biol Psychiatry 60:152–162. doi:10.1016/j.biopsych.2006.02.015
Li D, He L (2006) Association study of the G-protein signaling 4 (RGS4) and proline dehydrogenase (PRODH) genes with schizophrenia: a meta-analysis. Eur J Hum Genet 14:1130–1135. doi:10.1038/sj.ejhg.5201680
Guo S, Tang W, Shi Y et al (2006) RGS4 polymorphisms and risk of schizophrenia: an association study in Han Chinese plus meta-analysis. Neurosci Lett 406:122–127. doi:10.1016/j.neulet.2006.07.028
Allen NC, Bagade S, McQueen MB et al (2008) Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene database. Nat Genet 40:827–834
Stefanis NC, Trikalinos TA, Avramopoulos D et al (2008) Association of RGS4 variants with schizotypy and cognitive endophenotypes at the population level. Behav brain Funct 4:46. doi:10.1186/1744-9081-4-46
Kwapil TR, Barrantes-Vidal N (2014) Schizotypy: looking back and moving forward. Schizophr Bull 41(Suppl 2):S366–S373. doi:10.1093/schbul/sbu186
Barrantes-Vidal N, Gross GM, Sheinbaum T et al (2013) Positive and negative schizotypy are associated with prodromal and schizophrenia-spectrum symptoms. Schizophr Res 145:50–55. doi:10.1016/j.schres.2013.01.007
Barnett JH, McDougall F, Xu MK et al (2012) Childhood cognitive function and adult psychopathology: associations with psychotic and non-psychotic symptoms in the general population. Br J Psychiatry 201:124–130. doi:10.1192/bjp.bp.111.102053
Barrantes-Vidal N, Chun CA, Myin-Germeys I, Kwapil TR (2013) Psychometric schizotypy predicts psychotic-like, paranoid, and negative symptoms in daily life. J Abnorm Psychol 122:1077–1087. doi:10.1037/a0034793
Kwapil TR, Gross GM, Silvia PJ, Barrantes-Vidal N (2013) Prediction of psychopathology and functional impairment by positive and negative schizotypy in the Chapmans’ ten-year longitudinal study. J Abnorm Psychol 122:807–815. doi:10.1037/a0033759
Barrantes-Vidal N, Grant P, Kwapil TR (2015) The role of schizotypy in the study of the etiology of schizophrenia spectrum disorders. Schizophr Bull 41:S408–S416. doi:10.1093/schbul/sbu191
Stefanis NC, Hatzimanolis A, Avramopoulos D et al (2013) Variation in psychosis gene ZNF804A is associated with a refined schizotypy phenotype but not neurocognitive performance in a large young male population. Schizophr Bull 39:1252–1260. doi:10.1093/schbul/sbs110
Nenadic I, Maitra R, Basmanav FB et al (2015) ZNF804A genetic variation (rs1344706) affects brain grey but not white matter in schizophrenia and healthy subjects. Psychol Med 45:143–152. doi:10.1017/S0033291714001159
Prasad K, Chowdari K, Nimgaonkar V et al (2005) Genetic polymorphisms of the RGS4 and dorsolateral prefrontal cortex morphometry among first episode schizophrenia patients. Mol Psychiatry 10:213–219. doi:10.1038/sj.mp.4001562
Buckholtz JW, Meyer-lindenberg A, Honea RA et al (2007) Allelic variation in RGS4 impacts functional and structural connectivity in the human brain. J Neurosci 27:1584–1593. doi:10.1523/JNEUROSCI.5112-06.2007
de Castro-Catala M, Barrantes-Vidal N, Sheinbaum T et al (2015) COMT-by-sex interaction effect on psychosis proneness. Biomed Res Int 2015:1–7. doi:10.1155/2015/829237
Ros-Morente A, Vilagrà-Ruiz R, Rodríguez-Hansen G et al (2011) Proceso de Adaptación al Castellano de la Escala de Evaluación Comunitaria de Experiencias Psíquicas (CAPE). Actas Españolas Psiquiatr 39:95–105
Konings M, Bak M, Hanssen M et al (2006) Validity and reliability of the CAPE: a self-report instrument for the measurement of psychotic experiences in the general population. Acta Psychiatr Scand 114:55–61. doi:10.1111/j.1600-0447.2005.00741.x
Stephens M, Smith NJ, Donnelly P (2001) A new statistical method for haplotype reconstruction from population data. Am J Hum Genet 68:978–989. doi:10.1086/319501
van Os J, Linscott R, Myin-Germeys I et al (2009) A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol Med 39:179–195. doi:10.1017/S0033291708003814
Galderisi S, Merlotti E, Mucci A (2015) Neurobiological background of negative symptoms. Eur Arch Psychiatry Clin Neurosci. doi:10.1007/s00406-015-0590-4
Howes OD, Kapur S (2009) The dopamine hypothesis of schizophrenia: version III—the final common pathway. Schizophr Bull 35(3):549–562. doi:10.1093/schbul/sbp006
Erdely HA, Lahti RA, Lopez MB et al (2004) Regional expression of RGS4 mRNA in human brain. Eur J Neurosci 19:3125–3128. doi:10.1111/j.0953-816X.2004.03364.x
Lerner TN, Kreitzer AC (2012) RGS4 is required for dopaminergic control of striatal LTD and susceptibility to parkinsonian motor deficits. Neuron 73:347–359. doi:10.1016/j.neuron.2011.11.015
Grillet N, Dubreuil V, Dufour HD, Brunet J-F (2003) Dynamic expression of RGS4 in the developing nervous system and regulation by the neural type-specific transcription factor Phox2b. J Neurosci 23:10613–10621
Cheng Y-C, Scotting PJ, Hsu L-S et al (2013) Zebrafish rgs4 is essential for motility and axonogenesis mediated by Akt signaling. Cell Mol Life Sci 70:935–950. doi:10.1007/s00018-012-1178-z
Ripke S, Neale BM, Corvin A et al (2014) Biological insights from 108 schizophrenia-associated genetic loci. Nature 511:421–427. doi:10.1038/nature13595
Farrell MS, Werge T, Sklar P et al (2015) Evaluating historical candidate genes for schizophrenia. Mol Psychiatry 20:555–562. doi:10.1038/mp.2015.16
Acknowledgments
This work was funded by the Ministerio de Economía y Competitividad (PSI2011-30321-C02-01 and C02-02, and Red de Excelencia PSI2014-56303-REDT PROMOSAM: Investigación en Procesos, Mecanismos y Tratamientos Psicológicos para la Promoción de la Salud Mental), Fundació La Marató de TV3 (091110) and the Comissionat per a Universitats i Recerca of the Generalitat de Catalunya (2014SGR1070 and 2014SGR1636). N. Barrantes-Vidal is funded by the Academia Research Award (Institució Catalana de Recerca i Estudis Avançats; ICREA) from the Catalan Government.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
de Castro-Catala, M., Cristóbal-Narváez, P., Kwapil, T.R. et al. Association between RGS4 variants and psychotic-like experiences in nonclinical individuals. Eur Arch Psychiatry Clin Neurosci 267, 19–24 (2017). https://doi.org/10.1007/s00406-016-0676-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-016-0676-7