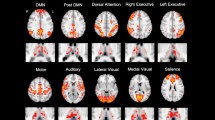
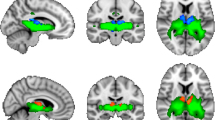
Abstract
Schizophrenia has been linked to disturbed connectivity between large-scale brain networks. Altered thalamocortical connectivity might be a major mechanism mediating regionally distributed dysfunction, yet it is only incompletely understood. We analysed functional magnetic resonance imaging data obtained during resting state from 22 DSM-IV schizophrenia patients and 22 matched healthy controls to directly assess the differences in thalamocortical functional connectivity. We identified significantly higher overall thalamocortical functional connectivity in patients, which was mostly accounted for by difference in thalamic connections to right ventrolateral prefrontal and bilateral secondary motor and sensory (superior temporal and lateral occipital) cortical areas. Voxelwise analysis showed group differences at the thalamic level to be mostly in medial and anterior thalamic nuclei and arising thalamocortical changes to be mostly due to higher positive correlations in prefrontal and superior temporal correlations, as well as absent negative correlations to sensory areas in patients. Our findings demonstrate that different types of thalamocortical dysfunction contribute to network alterations, including lack of inhibitory interaction attributed to the lack of significant negative thalamic/sensory cortical connections. These results emphasize the functional importance of the thalamus in the pathophysiology of schizophrenia.




Similar content being viewed by others
References
Achard S, Salvador R, Whitcher B, Suckling J, Bullmore E (2006) A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. J Neurosci 26(1):63–72. doi:10.1523/JNEUROSCI.3874-05.2006
Adcock RA, Dale C, Fisher M, Aldebot S, Genevsky A, Simpson GV, Nagarajan S, Vinogradov S (2009) When top-down meets bottom-up: auditory training enhances verbal memory in schizophrenia. Schizophr Bull 35(6):1132–1141. doi:10.1093/schbul/sbp068
Bassett DS, Bullmore E, Verchinski BA, Mattay VS, Weinberger DR, Meyer-Lindenberg A (2008) Hierarchical organization of human cortical networks in health and schizophrenia. J Neurosci 28(37):9239–9248. doi:10.1523/JNEUROSCI.1929-08.2008
Behrendt RP (2006) Dysregulation of thalamic sensory “transmission” in schizophrenia: neurochemical vulnerability to hallucinations. J Psychopharmacol 20(3):356–372. doi:10.1177/0269881105057696
Buchsbaum MS, Someya T, Teng CY, Abel L, Chin S, Najafi A, Haier RJ, Wu J, Bunney WE Jr (1996) PET and MRI of the thalamus in never-medicated patients with schizophrenia. Am J Psychiatry 153(2):191–199
Butler PD, Martinez A, Foxe JJ, Kim D, Zemon V, Silipo G, Mahoney J, Shpaner M, Jalbrzikowski M, Javitt DC (2007) Subcortical visual dysfunction in schizophrenia drives secondary cortical impairments. Brain 130(Pt 2):417–430. doi:10.1093/brain/awl233
Byne W, Hazlett EA, Buchsbaum MS, Kemether E (2009) The thalamus and schizophrenia: current status of research. Acta Neuropathol 117(4):347–368. doi:10.1007/s00401-008-0404-0
Byne W, Kidkardnee S, Tatusov A, Yiannoulos G, Buchsbaum MS, Haroutunian V (2006) Schizophrenia-associated reduction of neuronal and oligodendrocyte numbers in the anterior principal thalamic nucleus. Schizophr Res 85(1–3):245–253. doi:10.1016/j.schres.2006.03.029
Carter CS, Mintun M, Nichols T, Cohen JD (1997) Anterior cingulate gyrus dysfunction and selective attention deficits in schizophrenia: [15O]H2O PET study during single-trial Stroop task performance. Am J Psychiatry 154(12):1670–1675
Clark KA, Nuechterlein KH, Asarnow RF, Hamilton LS, Phillips OR, Hageman NS, Woods RP, Alger JR, Toga AW, Narr KL (2011) Mean diffusivity and fractional anisotropy as indicators of disease and genetic liability to schizophrenia. J Psychiatr Res 45(7):980–988. doi:10.1016/j.jpsychires.2011.01.006
Clinton SM, Meador-Woodruff JH (2004) Thalamic dysfunction in schizophrenia: neurochemical, neuropathological, and in vivo imaging abnormalities. Schizophr Res 69(2–3):237–253
Cole MW, Anticevic A, Repovs G, Barch D (2011) Variable global dysconnectivity and individual differences in schizophrenia. Biol Psychiatry 70(1):43–50. doi:10.1016/j.biopsych.2011.02.010
Corradi-Dell’acqua C, Tomelleri L, Bellani M, Rambaldelli G, Cerini R, Pozzi-Mucelli R, Balestrieri M, Tansella M, Brambilla P (2011) Thalamic-insular dysconnectivity in schizophrenia: evidence from structural equation modeling. Hum Brain Mapp. doi:10.1002/hbm.21246
Crespo-Facorro B, Paradiso S, Andreasen NC, O’Leary DS, Watkins GL, Boles Ponto LL, Hichwa RD (1999) Recalling word lists reveals “cognitive dysmetria” in schizophrenia: a positron emission tomography study. Am J Psychiatry 156(3):386–392
Dias EC, Butler PD, Hoptman MJ, Javitt DC (2011) Early sensory contributions to contextual encoding deficits in schizophrenia. Arch Gen Psychiatry 68(7):654–664. doi:10.1001/archgenpsychiatry.2011.17
Evans AC, Collins DL, Mills SR, Brown ED, Kelly RL, Peters TM (1993) 3D statistical neuroanatomical models from 305 MRI volumes, In: IEEE nuclear science symposium and medical imaging conference pp 1813–1817
Fornito A, Zalesky A, Pantelis C, Bullmore ET (2012) Schizophrenia, neuroimaging and connectomics. Neuroimage 62(4):2296–2314. doi:10.1016/j.neuroimage.2011.12.090
Fox MD, Snyder AZ, Zacks JM, Raichle ME (2006) Coherent spontaneous activity accounts for trial-to-trial variability in human evoked brain responses. Nat Neurosci 9(1):23–25. doi:10.1038/nn1616
Friedman T, Sehatpour P, Dias E, Perrin M, Javitt DC (2012) Differential relationships of mismatch negativity and visual p1 deficits to premorbid characteristics and functional outcome in schizophrenia. Biol Psychiatry 71(6):521–529. doi:10.1016/j.biopsych.2011.10.037
Friston KJ, Frith CD, Liddle PF, Frackowiak RS (1993) Functional connectivity: the principal-component analysis of large (PET) data sets. J Cereb Blood Flow Metab 13(1):5–14
Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, Turner R (1995) Analysis of fMRI time-series revisited. Neuroimage 2(1):45–53. doi:10.1006/nimg.1995.1007
Hampson M, Driesen N, Roth JK, Gore JC, Constable RT (2010) Functional connectivity between task-positive and task-negative brain areas and its relation to working memory performance. Magn Reson Imaging 28(8):1051–1057. doi:10.1016/j.mri.2010.03.021
Javitt DC (2009) When doors of perception close: bottom-up models of disrupted cognition in schizophrenia. Annu rev clin psychol 5:249–275. doi:10.1146/annurev.clinpsy.032408.153502
Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP (2008) Competition between functional brain networks mediates behavioral variability. Neuroimage 39(1):527–537. doi:10.1016/j.neuroimage.2007.08.008
Kemether EM, Buchsbaum MS, Byne W, Hazlett EA, Haznedar M, Brickman AM, Platholi J, Bloom R (2003) Magnetic resonance imaging of mediodorsal, pulvinar, and centromedian nuclei of the thalamus in patients with schizophrenia. Arch Gen Psychiatry 60(10):983–991. doi:10.1001/archpsyc.60.9.98360/10/983
Klingner CM, Hasler C, Brodoehl S, Axer H, Witte OW (2012) Perceptual plasticity is mediated by connectivity changes of the medial thalamic nucleus. Hum Brain Mapp. doi:10.1002/hbm.22074
Konick LC, Friedman L (2001) Meta-analysis of thalamic size in schizophrenia. Biol Psychiatry 49(1):28–38
Kubota M, Miyata J, Sasamoto A, Sugihara G, Yoshida H, Kawada R, Fujimoto S, Tanaka Y, Sawamoto N, Fukuyama H, Takahashi H, Murai T (2012) Thalamocortical disconnection in the orbitofrontal region associated with cortical thinning in schizophrenia. Arch Gen Psychiatry:1–10. doi:10.1001/archgenpsychiatry.2012.1023
Kuhn S, Gallinat J (2011) Resting-state brain activity in schizophrenia and major depression: a quantitative meta-analysis. Schizophr Bull. doi:10.1093/schbul/sbr151
Laurens KR, Kiehl KA, Ngan ET, Liddle PF (2005) Attention orienting dysfunction during salient novel stimulus processing in schizophrenia. Schizophr Res 75(2–3):159–171. doi:10.1016/j.schres.2004.12.010
Liang M, Zhou Y, Jiang T, Liu Z, Tian L, Liu H, Hao Y (2006) Widespread functional disconnectivity in schizophrenia with resting-state functional magnetic resonance imaging. Neuroreport 17(2):209–213
Liu Y, Liang M, Zhou Y, He Y, Hao Y, Song M, Yu C, Liu H, Liu Z, Jiang T (2008) Disrupted small-world networks in schizophrenia. Brain 131(Pt 4):945–961. doi:10.1093/brain/awn018
Liu Y, Yu C, Liang M, Li J, Tian L, Zhou Y, Qin W, Li K, Jiang T (2007) Whole brain functional connectivity in the early blind. Brain 130(Pt 8):2085–2096. doi:10.1093/brain/awm121
Lui S, Li T, Deng W, Jiang L, Wu Q, Tang H, Yue Q, Huang X, Chan RC, Collier DA, Meda SA, Pearlson G, Mechelli A, Sweeney JA, Gong Q (2010) Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by “resting state” functional magnetic resonance imaging. Arch Gen Psychiatry 67(8):783–792. doi:10.1001/archgenpsychiatry.2010.84
Lynall ME, Bassett DS, Kerwin R, McKenna PJ, Kitzbichler M, Muller U, Bullmore E (2010) Functional connectivity and brain networks in schizophrenia. J Neurosci 30(28):9477–9487. doi:10.1523/JNEUROSCI.0333-10.2010
Marenco S, Stein JL, Savostyanova AA, Sambataro F, Tan HY, Goldman AL, Verchinski BA, Barnett AS, Dickinson D, Apud JA, Callicott JH, Meyer-Lindenberg A, Weinberger DR (2012) Investigation of anatomical thalamo-cortical connectivity and FMRI activation in schizophrenia. Neuropsychopharmacology 37(2):499–507. doi:10.1038/npp.2011.215
Mingoia G, Wagner G, Langbein K, Maitra R, Smesny S, Dietzek M, Burmeister HP, Reichenbach JR, Schlosser RG, Gaser C, Sauer H, Nenadic I (2012) Default mode network activity in schizophrenia studied at resting state using probabilistic ICA. Schizophr Res 138(2–3):143–149. doi:10.1016/j.schres.2012.01.036
Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC (2009) Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry 66(8):811–822. doi:10.1001/archgenpsychiatry.2009.91
Mitelman SA, Brickman AM, Shihabuddin L, Newmark R, Chu KW, Buchsbaum MS (2005) Correlations between MRI-assessed volumes of the thalamus and cortical Brodmann’s areas in schizophrenia. Schizophr Res 75(2–3):265–281. doi:10.1016/j.schres.2004.10.014
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9(1):97–113
Pakkenberg B (1990) Pronounced reduction of total neuron number in mediodorsal thalamic nucleus and nucleus accumbens in schizophrenics. Arch Gen Psychiatry 47(11):1023–1028
Pakkenberg B (1992) The volume of the mediodorsal thalamic nucleus in treated and untreated schizophrenics. Schizophr Res 7(2):95–100
Popken GJ, Bunney WE Jr, Potkin SG, Jones EG (2000) Subnucleus-specific loss of neurons in medial thalamus of schizophrenics. Proc Natl Acad Sci USA 97(16):9276–9280. doi:10.1073/pnas.150243397150243397
Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2012) Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59(3):2142–2154. doi:10.1016/j.neuroimage.2011.10.018
Pynn LK, Desouza JF (2013) The function of efference copy signals: implications for symptoms of schizophrenia. Vision Res 76:124–133. doi:10.1016/j.visres.2012.10.019
Rissling AJ, Light GA (2010) Neurophysiological measures of sensory registration, stimulus discrimination, and selection in schizophrenia patients. Curr Top Behav Neurosci 4:283–309
Salami M, Itami C, Tsumoto T, Kimura F (2003) Change of conduction velocity by regional myelination yields constant latency irrespective of distance between thalamus and cortex. Proc Natl Acad Sci USA 100(10):6174–6179. doi:10.1073/pnas.0937380100
Salvador R, Suckling J, Coleman MR, Pickard JD, Menon D, Bullmore E (2005) Neurophysiological architecture of functional magnetic resonance images of human brain. Cereb Cortex 15(9):1332–1342. doi:10.1093/cercor/bhi016
Sambataro F, Blasi G, Fazio L, Caforio G, Taurisano P, Romano R, Di Giorgio A, Gelao B, Lo Bianco L, Papazacharias A, Popolizio T, Nardini M, Bertolino A (2010) Treatment with olanzapine is associated with modulation of the default mode network in patients with Schizophrenia. Neuropsychopharmacology 35(4):904–912. doi:10.1038/npp.2009.192
Scheeringa R, Petersson KM, Kleinschmidt A, Jensen O, Bastiaansen MC (2012) EEG alpha power modulation of fMRI resting state connectivity. Brain Connect. doi:10.1089/brain.2012.0088
Schmitt A, Hasan A, Gruber O, Falkai P (2011) Schizophrenia as a disorder of disconnectivity. Eur Arch Psychiatry Clin Neurosci 261(Suppl 2):S150–S154. doi:10.1007/s00406-011-0242-2
Sherman SM (2007) The thalamus is more than just a relay. Curr Opin Neurobiol 17(4):417–422. doi:10.1016/j.conb.2007.07.003
Sherman SM, Guillery RW (2002) The role of the thalamus in the flow of information to the cortex. Philos Trans R Soc Lond B Biol Sci 357(1428):1695–1708. doi:10.1098/rstb.2002.1161
Sherman SM, Guillery RW (2005) Exploring the Thalamus and its role in cortical function, 2nd edn. MIT Press, Cambridge, MA
Skudlarski P, Jagannathan K, Anderson K, Stevens MC, Calhoun VD, Skudlarska BA, Pearlson G (2010) Brain connectivity is not only lower but different in schizophrenia: a combined anatomical and functional approach. Biol Psychiatry 68(1):61–69. doi:10.1016/j.biopsych.2010.03.035
Smith MJ, Wang L, Cronenwett W, Mamah D, Barch DM, Csernansky JG (2011) Thalamic morphology in schizophrenia and schizoaffective disorder. J Psychiatr Res 45(3):378–385. doi:10.1016/j.jpsychires.2010.08.003
Steriade M (2006) Grouping of brain rhythms in corticothalamic systems. Neuroscience 137(4):1087–1106. doi:10.1016/j.neuroscience.2005.10.029
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15(1):273–289. doi:10.1006/nimg.2001.0978
Weissenbacher A, Kasess C, Gerstl F, Lanzenberger R, Moser E, Windischberger C (2009) Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. Neuroimage 47(4):1408–1416. doi:10.1016/j.neuroimage.2009.05.005
Welsh RC, Chen AC, Taylor SF (2010) Low-frequency BOLD fluctuations demonstrate altered thalamocortical connectivity in schizophrenia. Schizophr Bull 36(4):713–722. doi:10.1093/schbul/sbn145
Young KA, Manaye KF, Liang C, Hicks PB, German DC (2000) Reduced number of mediodorsal and anterior thalamic neurons in schizophrenia. Biol Psychiatry 47(11):944–953
Zhang D, Guo L, Hu X, Li K, Zhao Q, Liu T (2012) Increased cortico-subcortical functional connectivity in schizophrenia. Brain Imaging Behav 6(1):27–35. doi:10.1007/s11682-011-9138-z
Zikopoulos B, Barbas H (2007) Circuits for multisensory integration and attentional modulation through the prefrontal cortex and the thalamic reticular nucleus in primates. Rev Neurosci 18(6):417–438
Acknowledgments
This study was partially funded by a Young Scientist Grant of the Friedrich-Schiller-University of Jena (to I.N.; DRMF 21007087), and the EU (EUTwinsS, an RTN network, FP6: MRTN-CT-2006-035987; local PIs: I.N., and H.S.).
Conflict of interest
The authors declare that they have no relevant conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Klingner, C.M., Langbein, K., Dietzek, M. et al. Thalamocortical connectivity during resting state in schizophrenia. Eur Arch Psychiatry Clin Neurosci 264, 111–119 (2014). https://doi.org/10.1007/s00406-013-0417-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-013-0417-0