Abstract
Background
A molecular surrogate may exist for the clinical behaviour of juvenile nasopharyngeal angiofibroma (JNA).
Methods
In 9–14 cases, a ‘correlation’ of clinical behaviour with molecular expression (m-RNA expression through RT-PCR) of VEGF, FGF, PDGF, Ras, c-Myc, c-Kit and p53 was undertaken.
Results
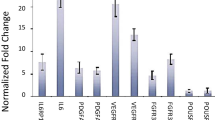
A comparison of the two extremes of expressions characterized some specific clinical phenotypes for every marker except c-Myc. A higher FGF was associated with post-adolescent presentation, smaller tumour size, enhanced haemorrhage and recurrence. A higher c-Kit was associated with adolescents, rapid growth, skull base involvement and recurrence. Enhanced Ras was associated with post-adolescence, smaller tumour size, skull base involvement and recurrence. Enhanced p53 and PDGF were associated with adolescents, early presentation and rapid progression. Higher VEGF expression was associated with skull base involvement and enhanced haemorrhage.
Conclusion
This study is currently the only evidence revealing a clinical molecular association in JNA and larger multicentric studies need to be performed to show a statistical significance.
Similar content being viewed by others
References
Schuon R, Brieger J, Heinrich UR, Roth Y, Szyfter W, Mann WJ (2007) Immunohistochemical analysis of growth mechanisms in juvenile angiofibroma. Eur Arch Otorhinolaryngol 264:389–394
Schiff M, Gonzalez AM, Ong M, Baird A (1992) Juvenile nasopharyngeal angiofibroma contain an angiogenic growth factor: basic FGF. Laryngoscope 102:940–945
Dillard DG, Cohen C, Muller S, Del Gaudio J, Reichman O, Parrish B et al (2000) Immunolocalization of activated transforming growth factor beta1 in juvenile nasopharyngeal angiofibroma. Arch Otolaryngol Head Neck Surg 126:723–725
Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF (1983) Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 219:983–985
Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M (1998) Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 92:735–745
Herzog B, Pellet-Many C, Britton G, Hartzoulakis B, Zachary IC (2011) VEGF binding to NRP1 is essential for VEGF stimulation of endothelial cell migration, complex formation between NRP1 and VEGFR2, and signaling via FAK Tyr407 phosphorylation. Mol Biol Cell 22:2766–2776
Palmer BF, Clegg DJ (2014) Oxygen sensing and metabolic homeostasis. Mol Cell Endocrinol 397:51–58
Matsumoto E, Sasaki S, Kinoshita H, Kito T, Ohta H, Konishi M et al (2013) Angiotensin II-induced cardiac hypertrophy and fibrosis are promoted in mice lacking Fgf16. Genes Cells 18:544–553
Xiao L, Du Y, Shen Y, He Y, Zhao H, Li Z (2012) TGF-beta 1 induced fibroblast proliferation is mediated by the FGF-2/ERK pathway. Front Biosci (Landmark Ed) 17:2667–2674
Warburton D (2012) Developmental responses to lung injury: repair or fibrosis. Fibrogenesis Tissue Repair 5:S2
Gupte VV, Ramasamy SK, Reddy R, Lee J, Weinreb PH, Violette SM et al (2009) Overexpression of fibroblast growth factor-10 during both inflammatory and fibrotic phases attenuates bleomycin induced pulmonary fibrosis in mice. Am J Respir Crit Care Med 180:424–436
Lemmon SK, Riley MC, Thomas KA, Hoover GA, Maciag T, Bradshaw RA (1982) Bovine fibroblast growth factor: comparison of brain and pituitary preparations. J Cell Biol 95:162–169
Thomas KA, Rios-Candelore M, Gimenez-Gallego G, DiSalvo J, Bennett C, Rodkey J et al (1985) Pure brain-derived acidic fibroblast growth factor is a potent angiogenic vascular endothelial cell mitogen with sequence homology to interleukin 1. Proc Natl Acad Sci USA 82:6409–6413
Schreiber AB, Kenney J, Kowalski WJ, Friesel R, Mehlman T, Maciag T (1985) Interaction of endothelial cell growth factor with heparin: characterization by receptor and antibody recognition. Proc Natl Acad Sci USA 82:6138–6142
Schreiber AB, Kenney J, Kowalski J, Thomas KA, Gimenez-Gallego G, Rios-Candelore M et al (1985) A unique family of endothelial cell polypeptide mitogens: the antigenic and receptor cross-reactivity of bovine endothelial cell growth factor, brain-derived acidic fibroblast growth factor, and eye-derived growth factor-II. J Cell Biol 101:1623–1626
Kumar V (2010) Robbins and coltran pathologic basis of disease. Elsevier, Beijing, pp 88–89 (ISBN: 978-1-4160-3121-5)
Demoulin JB, Montano-Almendras CP (2012) Platelet-derived growth factors and their receptors in normal and malignant hematopoiesis. Am J Blood Res 2:44–56
Andrae J, Gallini R, Betsholtz C (2008) Role of platelet-derived growth factors in physiology and medicine. Genes Dev 22:1276–1312
Hannink M, Donoghue DJ (1989) Structure and function of platelet-derived growth factor (PDGF) and related proteins. Biochim Biophys Acta 989:1–10
Heldin CH (1992) Structural and functional studies on platelet-derived growth factor. EMBO J 11:4251–4259
Zhang PJ, Weber R, Liang H, Pasha TL, LiVolsi VA (2003) Growth factors and receptors in juvenile nasopharyngeal angiofibroma and nasal polyps. Arch Pathol Lab Med 127:1480–1484
Pauli J, Gundelach R, Vanelli-Rees A, Rees G, Campbell C, Dubey S et al (2008) Juvenile nasopharyngeal angiofibroma: an immunohistochemical characterisation of the stromal cell. Pathology 40:396–400
Ribatti D, Ranieri G, Basile A, Azzariti A, Paradiso A, Vacca A (2012) Tumor endothelial markers as a target in cancer. Expert Opin Ther Targets 16:1215–1225
Downward J (2003) Targeting RAS signaling pathways in cancer therapy. Nat Rev Cancer 3:11–22
Lim KH, Baines AT, Fiordalisi JJ, Shipitsin M, Feig LA, Cox AD et al (2005) Activation of RalA is critical for Ras-induced tumorigenesis of human cells. Cancer Cell 7:533–545
Hamad NM, Elconin JH, Karnoub AE, Bai W, Rich JN, Abraham RT,.et al (2002) Distinct requirements for Ras oncogenesis in human versus mouse cells. Genes Dev 16:2045–2057
Paterson IC, Eveson JW, Prime SS (1996) Molecular changes in oral cancer may reflect aetiology and ethnic origin. Eur J Cancer B Oral Oncol 32:150–153
Mishra A, Mishra SC (2016) Changing trends in the incidence of juvenile nasopharyngeal angiofibroma: seven decades of experience at King George’s Medical University, Lucknow, India. J Laryngol Otol 130:363–368
Mishra A, Jaiswal R, Pandey A, Mishra SC (2018) Molecular interactions in juvenile nasopharyngeal angiofibroma: preliminary signature and relevant review. Eur Arch Otolaryngol (submitted)
Pandey P, Mishra A, Tripathi AM, Verma V, Trivedi R, Singh HP et al (2017) Current molecular profile of juvenile nasopharyngeal angiofibroma: first comprehensive study from India. Laryngoscope 127:e100–e106
Mishra A, Mishra SC, Verma V, Singh HP, Kumar S, Tripathi AM et al (2016) In defence of transpalatal, transpalatal-circumaxillary (transpterygopalatine) and transpalatal-circumaxillary-sublabial approaches to lateral extensions of juvenile nasopharyngeal angiofibroma. J Laryngol Otol 130:462–473
Radkowski D, McGill T, Healy GB, Ohlms L, Jones DT (1996) Angiofibroma: changes in staging and treatment. Arch Otolaryngol Head Neck Surg 122:122–129
Mishra SC, Shukla GK, Bhatia N, Pant MC (1989) A rational classification of angiofibromas of the postnasal space. J Laryngol Otol 103:912–916
Mishra A, Mishra SC, Pandey A (2017) Variations in molecular expressions of juvenile nasopharyngeal angiofibroma. J Laryngol Otol 131(9):752–759
Mishra A, Verma V (2018) Implication of embolization in residual disease in lateral extension of juvenile nasopharyngeal angiofibroma. J Oral Biol Craniofacial Res (submitted)
Saylam G, Yucel OT, Sungur A, Onerci M (2006) Proliferation, angiogenesis and hormonal markers in juvenile nasopharyngeal angiofibroma. Int J Pediatr Otorhinolaryngol 70:227–234
Brieger J, Wierzbicka M, Sokolov M, Roth Y, Szyfter W, Mann WJ (2004) Vessel density, proliferation and immunolocalization of vascular endothelial growth factor in juvenile nasopharyngeal angiofibromas. Arch Otolaryngol Head Neck Surg 130:727–731
Arbiser ZK, Arbiser JL, Cohen C, Gal AA (2001) Neuroendocrine lung tumors: grade correlates with proliferation but not angiogenesis. Mod Pathol 14:1195–1199
Juric G, Zarkovic N, Nola M, Tillian M, Jukić S (2001) The value of cell proliferation and angiogenesis in the prognostic assessment of ovarian granulosa cell tumors. Tumori 87:47–53
Mishra A, Singh V, Verma V, Pandey S, Trivedi R, Singh HP et al (2016) Current status and Clinical correlation of beta-catenin in juvenile nasopharyngeal angiofibroma. J Laryngol Otol 30:1–7
Profumo V, Gandellini P (2013) MicroRNAs: cobblestones on the road to cancer metastasis. Crit Rev Oncog 18:341–355
Pietras K, Pahler J, Bergers G, Hanahan D (2008) Functions of paracrine PDGF signalling in the proangiogenic tumor stroma revealed by pharmacological targeting. PLoS Med 5:e19
Song S, Ewald AJ, Stallcup W, Werb Z, Bergers G (2005) PDGFRbþ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat Cell Biol 7:870–879
Dong J, Grunstein J, Tejada M, Peale F, Frantz G, Liang WC et al (2004) VEGF null cells require PDGFR alpha signaling mediated stromal fibroblast recruitment for tumorigenesis. EMBO J 23:2800–2810
Nagai MA, Butugan O, Logullo A, Brentani MM (1996) Expression of growth factors, protooncogenes and p53 in nasopharyngeal angiofibromas. Laryngoscope 106:190–195
López-Martin A, Ballestín C, Garcia-Carbonero R, Castaño A, Lopez-Ríos F, López-Encuentra A et al (2007) Prognostic value of Kit expression in small cell lung cancer. Lung Cancer 56:405–413
Chau WK, Ip CK, Mak AS, Lai HC, Wong AS (2013) c-Kit mediates chemoresistance and tumor-initiating capacity of ovarian cancer cells through activation of Wnt/β-catenin-ATP-binding cassette G2 signaling. Oncogene 32:2767–2781
Wiesner C, Nabha SM, Dos Santos EB, Yamamoto H, Meng H, Melchior SW et al (2008) c-Kit and its ligand stem cell factor: potential contribution to prostate cancer bone metastasis. Neoplasia 10:996–1003
Cheng P, Chen H, Liu SR, Pu XY (2013) A ZC. SNPs in KIT and KITLG genes may be associated with oligospermia in Chinese population. Biomarkers 18:650–654
Marcu KB, Bossone SA, Patel AJ (1992) Myc function and regulation. Annu Rev Biochem 61:809–860
He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT et al (1998) Identification of c-MYC as a target of the APC pathway. Science 281:1509–1512
van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A et al (2002) The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 111:241–250
Sansom OJ, Reed KR, Hayes AJ, Ireland H, Brinkmann H, Newton IP et al (2004) Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation and migration. Genes Dev 18:1385–1390
Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW (1997) Oncogenic Ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88:593–602
Mitchell PJ, Perez-Nadales E, Malcolm DS, Lloyd AC (2003) Dissecting the contribution of p16(INK4A) and the Rb family to the Ras transformed phenotype. Mol Cell Biol 23:2530–2542
Coutinho CM, Bassini AS, Gutie´rrez LG, Butugan O, Kowalski LP, Brentani MM et al (1999) Genetic alterations in Ki-ras and Ha-ras genes in juvenile nasopharyngeal angiofibromas and head and neck cancer. Sao Paulo Med J 117:113–120
Schick B, Veldung B, Wemmert S, Jung V, Montenarh M, Meese E et al (2005) p53 and Her-2/neu in juvenile angiofibromas. Oncol Rep 13:453–457
Mishra A, Sachadeva M, Jain A, Shukla NM, Pandey A (2016) Human papilloma virus in juvenile nasopharyngeal angiofibroma: possible recent trend. Am J Otolaryngol Head Neck Med Surg 37:317–322
Acknowledgements
The principal author would like to acknowledge Praveen Pandey from CDRI for his efforts in generating the necessary laboratory data and while the basic molecular expression values have been published in the laryngoscope, the current study exclusively correlates the clinical parameters.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
This article is a part of the PhD work of Dr. Anupam Mishra.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mishra, A., Mishra, S.C., Tripathi, A.M. et al. Clinical correlation of molecular (VEGF, FGF, PDGF, c-Myc, c-Kit, Ras, p53) expression in juvenile nasopharyngeal angiofibroma. Eur Arch Otorhinolaryngol 275, 2719–2726 (2018). https://doi.org/10.1007/s00405-018-5110-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-018-5110-5