Abstract
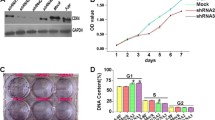
CDK8, a member of the transcriptional subtype of the cyclin-dependent kinases (CDKs) family, shows remarkable cancer tissue specific expression profile and rather more selective contribution to the regulation of gene expression levels involved in some signaling pathways. However, the effect of CDK8 on the malignant phenotype of human laryngeal squamous cell carcinoma (LSCC) cells and the potential molecular mechanisms remain unclear. In the present study, we evaluated the expression levels of CDK8 by quantitative real-time reverse-transcriptase polymerase chain reaction (qRT-PCR) and immunohistochemistry in tissue samples of 60 LSCC patients. Then we analyzed and correlated the results with clinicopathological features. We demonstrated that CDK8 was significantly overexpressed in LSCC tissues compared with normal controls, and this overexpression was correlated with lymph node metastasis and advanced clinical stages. Kaplan–Meier analysis showed that high expression levels of CDK8 miRNA significantly correlated with short OS survival. In addition, down-regulation of CDK8 using small interfering RNA(siRNA) reduced the proliferation and migration of LSCC in vitro. To explore the potential mechanism, we investigated the effect of CDK8 on Wnt signaling pathway and found that CDK8 was involved in the EMT progress by regulating β-catenin of the Wnt signaling. In summary, our data suggest for the first time that CDK8 appears to contribute to the malignant mechanism of LSCC and may represent a significant prognostic marker for LSCC patients.



Similar content being viewed by others
References
Lovato A (2015) Letter on the article “partial laryngectomy as salvage surgery after radiotherapy: oncological and functional outcomes and impact on quality of life. A retrospective study of 20 cases”. Eur Ann Otorhinolaryngol Head Neck Dis 132(3):175. doi:10.1016/j.anorl.2014.09.004
Rudolph E, Dyckhoff G, Becher H, Dietz A, Ramroth H (2011) Effects of tumour stage, comorbidity and therapy on survival of laryngeal cancer patients: a systematic review and a metaanalysis. Eur Arch Otorhinolaryngol 268:165–179. doi:10.1007/s00405-010-1395-8
Mulholland DJ, Dedhar S, Coetzee GA, Nelson CC (2005) Interaction of nuclear receptors with the Wnt/beta-catenin/Tcf signaling axis: Wnt you like to know? Endocr Rev 26:898–915. doi:10.1210/er.2003-0034
Katoh M, Katoh M (2007) Wnt signaling pathway and stem cell signaling network. Clin Cancer Res 13:4042–4045. doi:10.1158/1078-0432.CCR-06-2316
Kielman MF, Rindapaa M, Gaspar C et al (2002) Apc modulates embryonic stem-cell differentiation by controlling the dosage of beta-catenin signalling. Nat Genet 32:594–605. doi:10.1038/ng1045
Tsuchiya R, Yamamoto G, Nagoshi Y et al (2004) Expression of adenomatous polyposis coli (APC) in tumorigenesis of human oral squamous cell carcinoma. Oral Oncol 40:932–940. doi:10.1016/j.oraloncology.2004.04.011
Verras M, Sun Z (2006) Roles and regulation of Wnt signaling and beta-catenin in prostate cancer. Cancer Lett 237:22–32. doi:10.1016/j.canlet.2005.06.004
Guillen-Ahlers H (2008) Wnt signaling in renal cancer. Curr Drug Targets 9:591–600
Ito K, Lim AC, Salto-Tellez M, Motoda L, Osato M et al (2008) RUNX3 attenuates beta-catenin/T cell factors in intestinal tumorigenesis. Cancer Cell 14:226–237. doi:10.1016/j.ccr.2008.08.004
Scholer-Dahirel A, Schlabach MR, Loo A et al (2011) Maintenance of adenomatous polyposis coli (APC)-mutant colorectal cancer is dependent on Wnt/beta-catenin signaling. Proc Natl Acad Sci USA 108:17135–17140. doi:10.1073/pnas.1104182108
Mukherjee N, Bhattacharya N, Alam N, Roy A, Roychoudhury S, Panda CK (2012) Subtype-specific alterations of the Wnt signaling pathway in breast cancer. clinical and prognostic significance. Cancer Sci 103:210–220. doi:10.1111/j.1349-7006.2011.02131.x
Li M, Tian L, Wang L et al (2013) Down-regulation of miR-129-5p inhibits growth and induces apoptosis in laryngeal squamous cell carcinoma by targeting APC. PLoS One 8:e77829. doi:10.1371/journal.pone.0077829
Gupta S, Iljin K, Sara H et al (2010) FZD4 as a mediator of ERG oncogene induced WNT signaling and epithelial-to-mesenchymal transition in human prostate cancer cells. Cancer Res 70:6735–6745. doi:10.1158/0008-5472.CAN-10-0244
Bates RC, Mercurio AM (2005) The epithelial-mesenchymal transition (EMT) and colorectal cancer progression. Cancer Biol Ther 4:365–370
Christiansen JJ, Rajasekaran AK (2006) Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res 66:8319–8326. doi:10.1158/0008-5472.CAN-06-0410
Amin N, Vincan E (2012) The Wnt signaling pathways and cell adhesion. Front Biosci (Landmark Ed) 17:784–804
Yoda A, Kouike H, Okano H, Sawa H (2005) Components of the transcriptional mediator complex are required for asymmetric cell division in C. elegans. Development 132: 1885–1893. doi:10.1242/dev.01776
Kim S, Xu X, Hecht A, Boyer TG (2006) Mediator is a transducer of Wnt/beta-catenin signaling. J Biol Chem 281:14066–14075. doi:10.1074/jbc.M602696200
Carrera I, Janody F, Leeds N, Duveau F, Treisman JE (2008) Pygopus activates Wingless target gene transcription through the mediator complex subunits Med12 and Med13. Proc Natl Acad Sci USA 105:6644–6649. doi:10.1073/pnas.0709749105
Zhao J, Ramos R, Demma M (2013) CDK8 regulates E2F1 transcriptional activity through S375 phosphorylation. Oncogene 32:3520–3530. doi:10.1038/onc.2012.364
Liu XX, Li XJ, Zhang B et al (2011) MicroRNA-26b is under expressed in human breast cancer and induces cell apoptosis by targeting SLC7A11. FEBS Lett 585:1363–1367. doi:10.1016/j.febslet.2011.04.018
Morris EJ, Ji JY, Yang F et al (2008) E2F1 represses beta-catenin transcription and is antagonized by both pRB and CDK8. Nature 455:552–556. doi:10.1038/nature07310
He SB, Yuan Y, Wang L, Yu MJ, Zhu YB, Zhu XG (2011) Effects of cyclin-dependent kinase 8 specific siRNA on the proliferation and apoptosis of colon cancer cells. J Exp Clin Cancer Res 30:109. doi:10.1186/1756-9966-30-109
Malumbres M (2014) Cyclin-dependent kinases. Genome Biol 15:122
Chattopadhyay I, Singh A, Phukan R et al (2010) Genome-wide analysis of chromosomal alterations in patients with esophageal squamous cell carcinoma exposed to tobacco and betel quid from high-risk area in India. Mutat Res 696:130–138. doi:10.1016/j.mrgentox.2010.01.001
Kim MY, Han SI, Lim SC (2011) Roles of cyclin-dependent kinase 8 and β-catenin in the oncogenesis and progression of gastric adenocarcinoma. Int J Oncol 38:1375–1383. doi:10.3892/ijo.2011.948
Firestein R, Bass AJ, Kim SY et al (2008) CDK8 is a colorectal cancer oncogene that regulates beta-catenin activity. Nature 455:547–551. doi:10.1038/nature07179
Sheffer M, Bacolod MD, Zuk O et al (2009) Association of survival and disease progression with chromosomal instability: a genomic exploration of colorectal cancer. Proc Natl Acad Sci USA 106:7131–7136. doi:10.1073/pnas.0902232106
Mitra AP, Almal AA, George B et al (2006) The use of genetic programming in the analysis of quantitative gene expression profiles for identification of nodal status in bladder cancer. BMC Cancer 6:159. doi:10.1186/1471-2407-6-159
Kapoor A, Goldberg MS, Cumberland LK et al (2010) The histone variant macroH2A suppresses melanoma progression through regulation of CDK8. Nature 468:1105–1109. doi:10.1038/nature09590
Szilagyi Z, Gustafsson CM (2013) Emerging roles of Cdk8 in cell cycle control. Biochim Biophys Acta 1829:916–920. doi:10.1016/j.bbagrm.2013.04.010
Donner AJ, Szostek S, Hoover JM, Espinosa JM (2007) CDK8 is a stimulus specific positive coregulator of p53 target genes. Mol Cell 27:121–133. doi:10.1016/j.molcel.2007.05.026
Donner AJ, Ebmeier CC, Taatjes DJ, Espinosa JM (2010) CDK8 is a positive regulator of transcriptional elongation within the serum response network. Nat Struct Mol Biol 17:194–201. doi:10.1038/nsmb.1752
Galbraith MD, Allen MA, Bensard CL et al (2013) HIF1A employs CDK8-mediator to stimulate RNAPII elongation in response to hypoxia. Cell 153:1327–1339. doi:10.1016/j.cell.2013.04.048
Bancerek J, Poss ZC, Steinparzer I et al (2013) CDK8 kinase phosphorylates transcription factor STAT1 to selectively regulate the interferon response. Immunity 38:250–262. doi:10.1016/j.immuni.2012.10.017
Fryer CJ, White JB, Jones KA, Mastermind recruits CycC (2004) CDK8 to phosphorylate the Notch ICD and coordinate activation with turnover. Mol Cell 16:509–520. doi:10.1016/j.molcel.2004.10.014
Alarcon C, Zaromytidou AI, Xi Q et al (2009) Nuclear CDKs drive Smad transcriptional activation and turnover in BMP and TGF-beta pathways. Cell 139:757–769. doi:10.1016/j.cell.2009.09.035
Li XY, Luo QF, Wei CK, Li DF, Fang L (2013) siRNA-mediated silencing of CDK8 inhibits proliferation and growth in breast cancer cells. Int J Clin Exp Pathol 7:92–100
Gu W, Wang C, Li W et al (2013) Tumor-suppressive effects of CDK8 in endometrial cancer cells. Cell Cycle 12:987–999. doi:10.4161/cc.24003
Li J, Li X, Kong X, Luo Q, Zhang J, Fang L (2014) MiRNA-26b inhibits cellular proliferation by targeting CDK8 in breast cancer. Int J Clin Exp Med 7:558–565
Li XY, Luo QF, Wei CK, Li DF, Li J, Fang L (2014) MiRNA-107 inhibits proliferation and migration by targeting CDK8 in breast cancer. Int J Clin Exp Med 7:32–40
Xu W, Wang Z, Zhang W et al (2016) Mutated K-ras activates CDK8 to stimulate the epithelial-to-mesenchymal transition in pancreatic cancer in part via the Wnt/β-catenin signaling pathway. Cancer Lett 356:613–627. doi:10.1016/j.canlet.2014.10.008
Stemmer V, de Craene B, Berx G, Behrens J (2008) Snail promotes Wnt target gene expression and interacts with beta-catenin. Oncogene 27:5075–5080. doi:10.1038/onc.2008.140
Lee SY, Jeon HM, Ju MK et al (2012) Wnt/Snail signaling regulates cytochrome C oxidase and glucose metabolism. Cancer Res 72:3607–3617. doi:10.1158/0008-5472.CAN-12-0006
Leptin M (1991) Twist and snail as positive and negative regulators during Drosophila mesoderm development. Genes Dev 5:1568–1576
Yang J, Mani SA, Donaher JL et al (2004) Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 117:927–939. doi:10.1016/j.cell.2004.06.006
Reinhold MI, Kapadia RM, Liao Z, Naski MC (2006) The Wnt-inducible transcription factor Twist1 inhibits chondrogenesis. J Biol Chem 281:1381–1388. doi:10.1074/jbc.M504875200
Conacci-Sorrell M, Simcha I, Ben-Yedidia T, Blechman J, Savagner P, Ben-Ze’Ev A (2003) Autoregulation of E-cadherin expression by cadherin-cadherin interactions: the roles of beta-catenin signaling, Slug, and MAPK. J Cell Biol 163:847–857. doi:10.1083/jcb.200308162
Liang X, Zheng M, Jiang J, Zhu G, Yang J, Tang Y (2011) Hypoxia-inducible factor-1 alpha, in association with TWIST2 and SNIP1, is a critical prognostic factor in patients with tongue squamous cell carcinoma. Oral Oncol 47:92–97. doi:10.1016/j.oraloncology.2010.11.014
Kang Y, Massague J (2004) Epithelial-mesenchymal transitions: twist in development and metastasis. Cell 118:277–279. doi:10.1016/j.cell.2004.07.011
Thiery JP, Acloque H, Huang RY, Nieto MA (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139:871–890. doi:10.1016/j.cell.2009.11.007
Xue C, Plieth D, Venkov C, Xu C, Neilson EG (2003) The gatekeeper effect of epithelial-mesenchymal transition regulates the frequency of breast cancer metastasis. Cancer Res 63:3386–3394
Peinado H, Portillo F, Cano A (2004) Transcriptional regulation of cadherins during development and carcinogenesis. Int J Dev Biol 48:365–375. doi:10.1387/ijdb.041794hp
Lee JM, Dedhar S, Kalluri R, Thompson EW (2006) The epithelial–mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol 172(7):973–981. doi:10.1083/jcb.200601018
Acknowledgements
We thank the Department of Otorhinolaryngology at the Second Affiliated Hospital of Harbin Medical University for providing human laryngeal tissue samples.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
The study was funded by grants from the Heilongjiang Postdoctoral Fund (LBH-Z14162), the China Postdoctoral Science Fundation (2015M581476) and the National Natural Science Foundation of China (81602368).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the Ethics Committee of Harbin Medical University (No. 2,013,003) and was conducted in accordance with Resolution 196/96 of the Brazilian National Health Council. Informed consent was obtained from all individual participants included in the study.
Additional information
M. Li, X. Zhao contributed equally to this work.
Rights and permissions
About this article
Cite this article
Li, M., Zhao, X., Liu, Y. et al. Aberrant expression of CDK8 regulates the malignant phenotype and associated with poor prognosis in human laryngeal squamous cell carcinoma. Eur Arch Otorhinolaryngol 274, 2205–2213 (2017). https://doi.org/10.1007/s00405-017-4484-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-017-4484-0