Abstract
Vestibular evoked myogenic potentials (VEMPs) were measured in 22 unilateral Menière patients with monaural and binaural stimulation with 250 and 500 Hz tone bursts. For all measurement situations significantly lower VEMP amplitudes were on average measured at the affected side compared to the unaffected side. Unilateral Menière patients have, in contrast to normal subjects, asymmetric VEMPs, indicating a permanently affected vestibular (most likely otolith) system at the side of hearing loss. The diagnostic value of VEMP amplitude asymmetry measurement in individual patients is low, because of the large overlap of the VEMP amplitude asymmetry range for unilateral Menière patients with that for normal subjects.
Similar content being viewed by others
Introduction
Menière’s disease (MD) is an inner ear pathology characterised by episodic vertigo, hearing loss and tinnitus. The typical pathological finding in MD is an idiopathic endolymphatic hydrops. Apart from the cochlea, the saccule is the second most frequently affected site for hydrops [1, 2]. Most often mentioned complaints in MD are aural fullness and subjective problems with balance while standing and walking. Dysfunction of the saccule could explain these symptoms [3, 4]. However, a reliable diagnostic test to evaluate saccular function was until previous years not available. Standard clinical vestibular tests are limited to the evaluation of only one of the five vestibular organs, the horizontal semicircular canal.
A rather new method to measure the function of the otolith organs or the saccular function, which is still not standardly used in every clinic, is the non-invasive, well-tolerated, relatively simple vestibular evoked myogenic potential (VEMP) test, first described by Colebatch and Halmagyi [5, 6]. VEMPs are short latency electromyograms (EMG), evoked by loud acoustic stimuli and recorded using surface electrodes over the tonically contracted sternocleidomastoid (SCM) muscle.
Several studies in experimental animals and patients with peripheral audiovestibular lesions confirm the saccular origin of the response [6–9]. This vestibulocollic reflex is mediated by a pathway that includes the saccular macula, the inferior vestibular nerve and vestibulospinal tract [10]. Therefore, VEMP tests can be used to evaluate the function of the saccule and/or the inferior vestibular nerve [11]. Functional significance for this pathway is uncertain. In some more primitive vertebrates the saccule functions as a hearing organ [12]. Although the cochlea has replaced the saccule in mammals in this respect, it seems that the saccule has retained some acoustic sensitivity [7, 9].
The diagnostic utility of VEMPs has been investigated in several studies in patients with vestibulocochlear disorders, such as superior canal dehiscence, vestibular schwannoma and multiple sclerosis [13–17]. Studies on the diagnostic utility of VEMPs in Menière disease are sparse and the results inconsistent. Variation between the percentage of positive (decreased or increased) or absent VEMPs differs in several studies. [15, 18, 19]. This variation in results is probably due to the heterogeneity of patient populations and different detection methods. A more thorough evaluation of the diagnostic utility of VEMP in Menière disease patients is therefore justified. As saccular dysfunction is a pathophysiological feature of MD, we hypothesised that the VEMP amplitude is reduced in the affected ear. To test our hypothesis, we measured VEMPs at both sides in unilateral Menière patients with vertigo and instability complaints and used the contralateral unaffected ear as a reference.
One of the problems of the VEMP test is the long testing time in combination with substantial physical effort of the subjects. We therefore also compared the results of monaural and binaural stimulation, the latter allowing for shorter measurement time.
Materials and methods
Patients
Unilateral Menière patients under 65 years of age, in whom the disease was confirmed by the criteria of the Groningen Menière Definition (Table 1) were selected. Twenty-two unilateral Menière patients were included; 11 with an affected right ear and 11 with an affected left ear. 36% (N = 8) were female. The mean (SD) age was 53 ± 11 years. The mean duration of the disease was 4.8 (±3.2) years.
Preceding the VEMP test every patient underwent standard ear examination, pure tone audiometry, caloric tests and magnetic imaging of the cerebellopontine angle to exclude other audiovestibular diseases. Two weeks before the VEMP test, all antivertiginous medication was discontinued. Patients with neurological or musculoskeletal signs or symptoms and conductive hearing loss were excluded from the study.
Methods
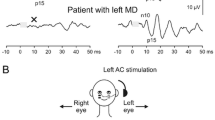
Hardware used for stimulus generation and response processing was: a RP2.1 real time processor, PA5 programmable attenuator, HB head phone driver, RA4LI electrode connector, RA4PA Medusa preamplifier, RA16 Medusa base station [manufacturer Tucker-Davis Technologies (TDT)]. Stimuli (100 dB nHL) were symmetrical 250 and 500 Hz tone bursts with a triangular envelope with 6, respectively, 3 ms rise and fall time (Fig. 1), generated with SigGenRP software (TDT) and presented with TDH39 headphones driven by two Philips PM5175 power amplifiers (one for each ear) at a rate of 5/s.
Responses were averaged 250 times with BioSigRP software (TDT) with filter settings 3 Hz–1 kHz
A non-inverting electrode was placed, as precisely as possible, at the midpoint of the sternocleidomastoid muscle on each side of the neck. The inverting electrodes were placed at the sternoclavicular junctions and the ground electrode was placed on the forehead. VEMPs were recorded on both sides with monaural and binaural stimulation. To keep the muscle tension constant during the test, the subjects pressed their forehead in sitting position against a cushioned bar and got visual feedback on muscle tension from a custom made LED array. A separate channel of the RP 2.1 processor was used to produce the root mean square (RMS) level of the left side amplified EMG signal, which was fed to the array. The p13-n23 amplitudes of the VEMP were measured.
Statistical methods
VEMP amplitudes of Menière ears and contralateral unaffected ears were compared using paired sample t tests. Correlations were expressed as Spearman’s correlation coefficient. All reported probability (p) values are two-sided and a value below 0.05 was considered statistically significant.
Results
General characteristics
The perceptive hearing loss in the Menière ears was at least 60 dB when summed over the worst three octaves. The contralateral (healthy) ears showed normal hearing levels or only a slight hearing loss at the high frequencies (Fig. 2). Almost all Menière ears revealed a reduced caloric response; excitability of the Menière ear was on average 45 ± 20% of that of the unaffected ear.
VEMP measurements
Significantly lower VEMP amplitudes were on average measured at the side of the affected ear for both stimulus frequencies (250 and 500 Hz) and binaural (B) as well as monaural (M) stimuli (Fig. 3).
In Fig. 4 the relation between mean hearing loss in the Menière ear and the VEMP amplitude measured at the side of that ear for monaural stimulation with 500 Hz tone bursts is shown.
Relation between mean hearing loss in the Menière ear and the VEMP amplitude measured at the side of that ear. Mean hearing loss is the average loss at 250 up to and including 8,000 Hz. The Menière ear was monaurally stimulated with 500 Hz tone bursts. The dashed line is a least squares fit with a second order curve
Figure 5 gives results for individual ears for binaural stimulation with 500 Hz.
Discussion
Side difference in unilateral Menière patients
Averaged over a group of normal subjects VEMP amplitudes measured at the right and the left sides are equal, both for binaural and monaural stimulation at the side of measurement [20, 21]. In other words, there is no side preference in normal subjects. This is not true for Menière patients; as can be seen in Fig. 3, the average VEMP amplitude at the affected side is significantly lower than that at the unaffected side. The smallest p value is found for monaural stimulation with 500 Hz tone bursts.
Side difference is a standard measure of vestibular function in patients, for instance as the outcome of a caloric test.
Averaged over all four stimulus situations (M250, M500, B250, B500), the ratio R = (average Aa)/(average Au) is 0.67 (Aa and Au are the amplitudes for the affected and unaffected ears, respectively).
Rauch et al. [19; Fig. 4] found a similar value (R = 0.61) for monaural stimulation with 500 Hz tone bursts in 34 unilateral Menière patients, but for stimulation with 250 Hz tone bursts they found R = 1.
Table 3 in Young et al. [18] gives an average value of 0.23 for the IAD ratio for 40 patients with unilateral definite MD, stimulated with 500 Hz tone bursts [IAD = interaural amplitude difference = (Au − Aa)/(Au + Aa)]. Although the average IAD ratio cannot directly be compared with R, also the result for the average IAD corresponds with a significantly lower average VEMP amplitude in the affected ear. The reduced VEMP amplitude at the affected side points toward a permanently affected otolith system in unilateral Menière patients at the side of the Menière ear. The affection of the cochlea and (part of) the vestibular system are related (Fig. 4): smaller VEMP amplitudes are found for larger hearing losses. Young et al. [18] found a significant relation between the stage of MD and the average VEMP IAD: the average IAD increased from stage 1 to stage 4. To stage the disease, the guidelines [22] of the American Academy of Otolaryngology—Head and Neck Surgery were followed. According to these guidelines, as larger hearing loss corresponds with a later stage, Young et al. found that larger VEMP amplitude asymmetry is measured in Menière patients with larger hearing losses, which corresponds with the relation shown in Fig. 4.
In Menière patients with drop attacks, these attacks are thought to be caused by an “otolithic catastrophe” [23]. Timmer et al. [24] found significantly more absent VEMPs in patients with drop attacks and although the precise histopathological and physiological explanations for drop attacks are not known, these findings also support the assumption that the otolith system is affected in Menière patients.
Binaural versus monaural stimulation
It can be seen in Fig. 3 that binaural or monaural stimulation produce on an average the same VEMP amplitude in the unaffected ears, both for 250 and 500 Hz stimuli. This result corresponds with that of Brantberg and Fransson [21], who found for 23 normal subjects that the ipsilateral response to monaural clicks was similar to the response to binaural clicks. Wang and Young [20], however, found for binaural stimulation an average VEMP amplitude of 83% of that for monaural stimulation with 500 Hz tone bursts in 14 healthy subjects.
As can be seen in Fig. 3, monaural stimulation produces larger average VEMP amplitude differences between the affected and the unaffected ear than binaural stimulation. This is in accordance with the result of Wang and Young [20], who found a larger median IAD ratio for monaural stimulation compared to simultaneous binaural stimulation in 12 Menière patients.
Binaural stimulation, on the other hand, has the advantage of substantially shorter measurement time and thus less muscular effort, which is of importance in particular for older patients.
Diagnostic value
VEMP amplitude differences in normal individuals can be large. According to Murofushi and Kaga [25], VEMP amplitude asymmetry should exceed 34% to be pathological. Asymmetry is defined as 100(Au − Aa)/(Au + Aa), in which Au and Aa are the amplitudes measured at the unaffected and affected side, respectively. Welgampola and Colebatch [26] gave values for the asymmetry range for click stimulation in normal subjects. This range (all measured values are smaller) is about 30% for subjects between 20 and 40 years of age, 45% for 40–60 years and even larger for subjects older than 60 years. Table 3 in Wang and Young [20] gives a normal asymmetry range of 35% for binaural stimulation with 500 Hz tone bursts in young subjects. Taking into account that two standard deviations is in general a smaller value than the total range it is well-founded, considering the above given values, to take 35% as the limit for normal asymmetry for subjects between 40 and 60 years of age, the age range of our Menière patients. This yields a approximate value of 2 for Aa/Au as the boundary between normal and pathological.
This boundary is shown in Fig. 5. And although in 18 of the 22 Menière patients Au is equal to or larger than Aa, only 3 patients can be classified as having a pathological VEMP amplitude asymmetry. So, while measuring VEMP, amplitude ratio Aa/Au is used as a test in unilateral Menière patients to confirm vestibular pathology of the affected ear; our results for binaural stimulation with 500 Hz tone bursts yield a test sensitivity of 14% (3/22).
Conclusion
In contrast to normal subjects, unilateral Menière patients have on average smaller VEMPs at the affected side compared to the unaffected side. The diagnostic value of VEMP amplitude asymmetry measurement in individual patients is low, because of the large overlap of the VEMP amplitude asymmetry range for unilateral Menière patients with that for normal subjects.
References
Rauch SD (2001) Vestibular histopathology of the human temporal bone. What can we learn? Ann NY Acad Sci 942:25–33
Okuno T, Sando I (1987) Localization, frequency and severity of endolymphatic hydrops and the pathology of the labyrinthine membrane in Meniere’s disease. Ann Otol Rhinol Laryngol 96:438–445
Black FO (1982) Vestibular function assessment in patients with Meniere’s disease: the vestibulospinal system. Laryngoscope 92:1419–1436
Van de Heyning PH, Wuyts FL, Claes J, Koekelkoren E, van Laer C, Valcke H (1997) Definition, classification and reporting of Meniere’s disease and its symptoms. Acta Otolaryngol Suppl 526:5–9
Colebatch JG, Halmagyi GM, Skuse NF (1994) Myogenic potentials generated by a click-evoked vestibulocollic reflex. J Neurol Neurosurg Psychiatry 57:190–197
Colebatch JG, Halmagyi GM (1992) Vestibular evoked potentials in human neck muscles before and after unilateral vestibular deafferentation. Neurology 42:1635–1636
McCue MP, Guinan JJ Jr (1995) Spontaneous activity and frequency of acoustically responsive vestibular afferents in cat. J Neurophysiol 74:1563–1572
Didier A, Cazals Y (1989) Acoustic responses recorded from the saccular bundle on the eight nerve of the guinea pig. Hear Res 37:123–127
Murofushi T, Curthoys IS, Topple AN, Colebatch JG, Halmagyi GM (1995) Response of guinea pig primary vestibular neurons to clicks. Exp Brain Res 103:174–178
Ito K, Ishimoto S, Murofushi T (2001) Narrow internal auditory meatus; an idiopathic case confirming the origin and pathway of vestibular evoked myogenic potentials in humans. Arch Otolaryngol Head Neck Surg 127:275–278
Colebatch JC (2001) Vestibular evoked potentials. Curr Opin Neurol 14:21–26
Popper A, Platt C, Saidal W (1982) Acoustic functions in the fish ear. Trends Neurosci 5:276–280
Ozeki H, Matsuzaki M, Murofushi T (1999) Vestibular evoked myogenic potentials in patients with bilateral profound hearing loss. ORL J Otorhinolaryngol Relat Spec 61:80–83
Murofushi T, Matsuzaki M, Mizuno M (1998) Vestibular evoked myogenic potentials in patients with acoustic neuromas. Arch Otolaryngol Head Neck Surg 124:509–512
De Waele C, Huy PTB, Diard JP, Freyss G, Vidal PP (1999) Saccular dysfunction in Meniere’s disease. Am J Otol 20:223–232
Minor LB, Cremer PD, Carey JP, Della Santina CC, Streubel SO, Weg N (2001) Symptoms and signs in superior canal dehiscence syndrome. Ann NY Acad Sci 942:259–273
Shimizu K, Murofushi T, Sakurai M, Halmagyi M (2000) Vestibular evoked myogenic potentials in multiple sclerosis. J Neurol Neurosurg Psychiatry 69:276–277
Young YH, Huang TW, Cheng PW (2003) Assessing the stage of Meniere’s disease using vestibular evoked myogenic potentials. Arch Otolaryngol Head Neck Surg 129:815–818
Rauch SD, Zhou G, Kujawa SG, Guinan JJ, Herrmann BS (2004) Vestibular evoked myogenic potentials show altered tuning in patients with Meniere’s disease. Otol Neurotol 25:333–338
Wang SJ, Young YH (2003) Vestibular evoked myogenic potentials using simultaneous binaural acoustic stimulation. Hear Res 185:43–48
Brantberg K, Fransson P-A (2001) Symmetry measures of vestibular evoked myogenic potentials using objective detection criteria. Scand Audiol 30:189–196
American Academy of Otolaryngology—Head, Neck Surgery (1995) Committee on hearing and equilibrium guidelines for the diagnosis and evaluation of therapy in Menière’s disease. Otolarynol Head Neck Surg 113:181–185
Tumarkin A (1936) The otolithic catastrophe. A new syndrome. BMJ 1:174–177
Timmer FCA, Zhou G, Guinan JJ, Kujawa SG, Herrmann BS, Rauch SD (2006) Vestibular evoked myogenic potential (VEMP) in patients with Menière’s disease with drop attacks. Laryngoscope 116:776–779
Murofushi T, Kaga K (2009) Vestibular evoked myogenic potentials: its basics and clinical applications. Springer, Tokyo, p 30
Welgampola MS, Colebatch JG (2001) Vestibulocollic reflexes; normal values and the effect of age. Clin Neurophysiol 112:1971–1979
Conflict of interest
None.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Kingma, C.M., Wit, H.P. Asymmetric vestibular evoked myogenic potentials in unilateral Menière patients. Eur Arch Otorhinolaryngol 268, 57–61 (2011). https://doi.org/10.1007/s00405-010-1345-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-010-1345-5