Abstract
Purpose
Melatonin is an important factor in regulating numerous processes in human female reproduction. The aim of the present study was to compare melatonin levels in the follicular fluid (FF) of ovarian hyperstimulation syndrome (OHSS) women with those of non-OHSS women undergoing in vitro fertilization (IVF)-embryo transfer and to evaluate the relationship between FF melatonin levels and IVF outcomes in these women.
Methods
We determined FF melatonin levels in 20 OHSS women and 23 non-OHSS women on oocyte retrieval day.
Results
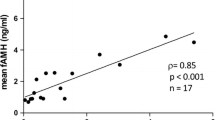
OHSS patients had significantly higher melatonin levels as compared to the non-OHSS women (P < 0.001). In addition, melatonin levels of the patients were significantly positively correlated with antral follicle count (AFC), serum anti-Müllerian hormone (AMH) levels, serum estradiol (E2) levels on human chorionic gonadotropin (HCG) administration day, number of retrieved oocytes, total fertilized oocytes, normally fertilized oocytes, cleaved zygotes, top quality embryos on day 3, blastocysts obtained and embryos suitable for transplantation (day 3 embryos + day 5/6 blastocysts) (P < 0.05). While, the intrafollicular melatonin levels were significantly negatively correlated with age, basal serum follicle-stimulating hormone (FSH) levels, serum FSH levels on HCG administration day (P < 0.01). Since younger women with more AFC, higher AMH levels, higher serum E2 levels and larger number of retrieved oocytes are much easier to encounter OHSS, while FF melatonin levels are significantly correlated with these five indices in our study, we propose that intrafollicular melatonin concentration can also be an important predictor of OHSS.
Conclusions
This is the first demonstration that FF melatonin levels were significantly higher in OHSS patients than in non-OHSS group and FF melatonin levels may serve as an important predictor of OHSS.

Similar content being viewed by others
References
Li HW, Lee VC, Lau EY et al (2014) Cumulative live-birth rate in women with polycystic ovary syndrome or isolated polycystic ovaries undergoing in-vitro fertilization treatment. J Assist Reprod Genet 31(2):205–211
Nastri CO, Ferriani RA, Rocha IA et al (2010) Ovarian hyperstimulation syndrome: pathophysiology and prevention. J Assist Reprod Genet 27(2):121–128
Mocanu E, Redmond ML, Hennelly B et al (2007) Odds of ovarian hyperstimulation syndrome (OHSS)-time for reassessment. Hum Fertil (Camb) 10(3):175–181
The Practice Committee of the American Society for Reproductive Medicine (HSRM) (2008) Ovarian hyperstimulation syndrome. Fertil Steril 90(Suppl. 5):S188–S193
Practice Committee, of American, Society for, Reproductive M (2008) Ovarian hyperstimulation syndrome. Fertil Steril 90(8):S188–S193
Geva E, Jaffe RB (2000) Role of vascular endothelial growth factor in ovarian physiology and pathology. Fertil Steril 74(3):429–438
Levin ER, Rosen GF, Cassidenti DL et al (1998) Role of vascular endothelial cell growth factor in ovarian hyperstimulation syndrome. J Clin Investig 102(11):1978–1985
Cui LL, Yang G, Pan J et al (2011) Tumor necrosis factor α knockout increases fertility of mice. Theriogenology 75(5):867–876
Guo T, Zhang L, Cheng D et al (2015) Low-density lipoprotein receptor affects the fertility of female mice. Reprod Fertil Dev 27(8):1222–1232
Guo SJ, Yan XY, Shi FF et al (2018) Expression and distribution of the zinc finger protein, SNAI3, in mouse ovaries and pre-implantation embryos. J Reprod Dev 64(2):179–186
Liu M, Xie S, Zhou J (2018) Use of animal models for the imaging and quantification of angiogenesis. Exp Anim 67(1):1–6
Papanikolaou EG, Pozzobon C, Kolibianakis EM et al (2006) Incidence and prediction of ovarian hyperstimulation syndrome in women undergoing gonadotropin-releasing hormone antagonist in vitro fertilization cycles. Fertil Steril 85(1):112–120
Reiter RJ, Tan DX, Manchester LC et al (2009) Melatonin and reproduction revisited. Biol Reprod 81(3):445–456
Tamura H, Takasaki A, Taketani T et al (2012) The role of melatonin as an antioxidant in the follicle. J Ovarian Res 5(5):5
Malpaux B, Migaud M, Tricoire H et al (2001) Biology of mammalian photoperiodism and the critical role of the pineal gland and melatonin. J Biol Rhythms 16(4):336–347
Kauffman AS, Clifton DK, Steiner RA (2007) Emerging ideas about kisspeptin-GPR54 signaling in the neuroendocrine regulation of reproduction. Trends Neurosci 30(10):504–511
Brzezinski A, Seibel MM, Lynch HJ et al (1987) Melatonin in human preovulatory follicular fluid. J Clin Endocrinol Metab 64(4):865–867
Rönnberg L, Kauppila A, Leppäluoto J et al (1990) Circadian and seasonal variation in human preovulatory follicular fluid melatonin concentration. J Clin Endocrinol Metab 71(2):492–496
Nakamura Y, Tamura H, Takayama H et al (2003) Increased endogenous level of melatonin inpreovulatory human follicles does not directly influence progesterone production. Fertil Steril 80(4):1012–1016
Reiter RJ, Tan DX, Fuentes-Broto L (2010) Melatonin: a multitasking molecule. Prog Brain Res 181(10):127–151
Tamura H, Nakamura Y, Korkmaz A (2009) Melatonin and the ovary: physiological and pathological implications. Fertil Steril 92(1):328–343
Whelan JG III, Vlahos NF (2000) The ovarian hyperstimulation syndrome. Fertil Steril 73(5):883–896
Golan A, Ron-el R, Herman A (1989) Ovarian hyperstimulation syndrome: an update review. Obstet Gynecol Surv 44(6):430–440
Brinsden PR (1999) A testbook of in vitro fertilization and assisted reproduction. The Parthenon Publishing Group Inc, New York
Gardner PK, Lane M, Stevens J et al (2000) Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril 73(6):1155–1158
Humaidan P, Quartarolo J, Papanikolaou EG (2010) Preventing ovarian hyperstimulation syndrome: guidance for the clinician. Fertil Steril 94(2):389–400
Tamura H, Taksaki A, Taketani T et al (2013) Melatonin as a free radical scavenger in the ovarian follicle. Endocr J 60(1):1–13
Nakamura Y, Smith M, Krishna A (1987) Increased number of mast cells in the dominant follicle of the cow: relationships among luteal, strenal and hilar regions. Biol Reprod 37(3):546–549
Brannstrom M, Mayrhofer G, Robertson SA (1993) Localization of leucocyte subsets in the rat ovary during the periovulatory period. Biol Reprod 48(2):277–286
Xie W, Zhou J (2018) Aberrant regulation of autophagy in mammalian diseases. Biol Lett 14(1):1–7
Gupta RK, Mille KP, Babus JK et al (2006) Methoxychlor inhibits growth and induces atresia of antral follicles through an oxidative stress pathway. Toxicol Sci 93(2):382–389
Korzekwa AJ, Okuda K, Woclawek-Potocka I et al (2006) Nitric oxide induces apoptosis in bovine luteal cells. J Reprod Dev 52(3):353–361
Lanoix D, Lacasse AA, Reiter RJ et al (2012) Melatonin: the smart killer: the human trophoblast as a model. Mol Cell Endocrinol 348(1):22–28
Zavodnik IB, Domanski AV, Lapshina EA (2006) Melatonin directly scavenges free radicals generated in red blood cells and a cell-free system: chemiluminescence measurements and theoretical calculations. Life Sci 79(4):391–400
Weenen C, Laven JS, Von Bergh AR et al (2004) Anti-Müllerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod 10(2):77–83
Waldhauser F, Weiszenbacher G, Tatzer E et al (1988) Alterations in nocturnal serum melatonin levels in humans with growth and aging. J Clin Endocrinol Metab 66(3):648–652
Vakkuri O, Kivelä A, Leppäluoto J et al (1996) Decrease in melatonin precedes follicle-stimulating hormone increase during perimenopause. Eur J Endocrinol 135(2):188–192
Pappolla MA, Chyan YJ, Poeggeler B et al (2000) An assessment of the antioxidant and the antiamyloidogenic properties of melatonin: implications for Alzheimer’s disease. J Neural Transm 107(2):203–231
Bonilla E, Valero N, Chacin-Bonilla L (2004) Melatonin and viral infections. J Pineal Res 36(2):73–79
Hussain SA, Khadim HM, Khalaf BH et al (2006) Effects of melatonin and zinc on glycemic control in type 2 diabetic patients poorly controlled with metformin. Saudi Med J 27(10):1483–1488
Lissoni P (2007) Biochemotherapy with standard chemotherapies plus the pineal hormone melatonin in the treatment of advanced solid neoplasms. Pathol Biol 55(3–4):201–204
Tamura H, Takasaki A, Miwa I et al (2008) Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J Pineal Res 44(3):280–287
Funding
This work was supported by a grant from the Major Program of the National Natural Science Foundation of China (81490743) to Z.-J.C.; by grants from National Key R&D Program of China (2017YFC1001403) and the National Natural Science Foundation of China (NSFC: 31671199 and 31871512) to C.Z.; and by the Shanghai Commission of Science and Technology (17DZ2271100).
Author information
Authors and Affiliations
Contributions
CZ: project development and editing, MZ: tissue collection, data analysis, manuscript writing and project development, GZ: tissue collection, JT: tissue collection, W-PL: project development, Z-JC: project development.
Corresponding author
Ethics declarations
Conflict of interest
All of the authors declare that they have no competing interests.
Ethical approval
All procedures performed in this study involving were in accordance with the ethical standards of the institutional research committee (no. 2015030308) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Zheng, M., Zuo, G., Tong, J. et al. Intrafollicular melatonin concentration is elevated in patients with ovarian hyperstimulation syndrome (OHSS) and can serve as an important predictor of OHSS. Arch Gynecol Obstet 299, 1151–1158 (2019). https://doi.org/10.1007/s00404-018-4994-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-018-4994-z