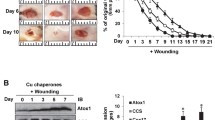
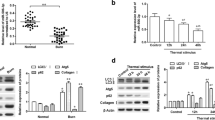
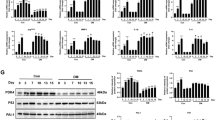
Abstract
Mitochondria are the major source of reactive oxygen species (ROS) in fibroblasts which are thought to be crucial regulators of wound healing with a potential to affect the expression of nuclear genes involved in this process. ROS generated by mitochondria are involved in all stages of tissue repair process but the regulation of ROS-generating system in fibroblasts still remains poorly understood. The purpose of this study was to better understand molecular mechanisms of how the regulation of ROS levels generated by mitochondria may influence the process of wound repair. Cybrid model system of mtDNA variations was used to study the functional consequences of altered ROS levels on wound healing responses in a uniform nuclear background of cultured ρ0 fibroblasts. Mitochondrial ROS in cybrids were modulated by antioxidants that quench ROS to examine their ability to close the wound. Real-time PCR arrays were used to investigate whether ROS generated by specific mtDNA variants have the ability to alter expression of some key nuclear-encoded genes central to the wound healing response and oxidative stress. Our data suggest levels of mitochondrial ROS affect expression of some nuclear encoded genes central to wound healing response and oxidative stress and modulation of mitochondrial ROS by antioxidants positively affects in vitro process of wound closure. Thus, regulation of mitochondrial ROS-generating system in fibroblasts can be used as effective natural redox-based strategy to help treat non-healing wounds.





Similar content being viewed by others
References
Al-Qattan MM, Abd-Elwahed MM, Hawary K, Arafah MM, Shier MK (2015) Myofibroblast expression in skin wounds is enhanced by collagen III suppression. Biomed Res Int. 2015:958695
Bayona-Bafaluy MP, Acin-Perez R, Mullikin JC, Park JS, Moreno-Loshuertos R, Hu P, Perez-Martos A, Fernandez-Silva P, Bai Y, Enriquez JA (2003) Revisiting the mouse mitochondrial DNA sequence. Nucleic Acids Res 31(18):5349–5355
Cerutti PA, Trump BF (1991) Inflammation and oxidative stress in carcinogenesis. Cancer cells—a monthly review 3(1):1–7
D’Autreaux B, Toledano MB (2007) ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol 8(10):813–824
De la Fuente M, Carazo M, Correa R, Del Rio M (2000) Changes in macrophage and lymphocyte functions in guinea-pigs after different amounts of vitamin E ingestion. Br J Nutr 84(1):25–29
Deonarine K, Panelli MC, Stashower ME, Jin P, Smith K, Slade HB, Norwood C, Wang E, Marincola FM, Stroncek DF (2007) Gene expression profiling of cutaneous wound healing. Journal of Translational Medicine 5:11
Eroglu I, Gokce EH, Tsapis N, Tanriverdi ST, Gokce G, Fattal E, Ozer O (2015) Evaluation of characteristics and in vitro antioxidant properties of RSV loaded hyaluronic acid-DPPC microparticles as a wound healing system. Coll Surf B Biointerfaces 126:50–57
Galeano M, Torre V, Deodato B, Campo GM, Colonna M, Sturiale A, Squadrito F, Cavallari V, Cucinotta D, Buemi M, Altavilla D (2001) Raxofelast, a hydrophilic vitamin E-like antioxidant, stimulates wound healing in genetically diabetic mice. Surgery 129(4):467–477
Greaves LC, Reeve AK, Taylor RW, Turnbull DM (2012) Mitochondrial DNA and disease. J Pathol 226(2):274–286
Guo S, Dipietro LA (2010) Factors affecting wound healing. J Dent Res 89(3):219–229
Hobson R (2014) Vitamin E and wound healing: an evidence-based review. Int Wound J
Hornig-Do HT, von Kleist-Retzow JC, Lanz K, Wickenhauser C, Kudin AP, Kunz WS, Wiesner RJ, Schauen M (2007) Human epidermal keratinocytes accumulate superoxide due to low activity of Mn-SOD, leading to mitochondrial functional impairment. J Invest Dermatol 127(5):1084–1093
James TJ, Hughes MA, Cherry GW, Taylor RP (2003) Evidence of oxidative stress in chronic venous ulcers. Wound Repair Regen 11(3):172–176
Jandova J, Eshaghian A, Shi M, Li M, King LE, Janda J, Sligh JE (2012) Identification of an mtDNA Mutation Hot Spot in UV-Induced Mouse Skin Tumors Producing Altered Cellular Biochemistry. J Investig Dermatol 132(2):421–428
Jandova J, Janda J, Sligh JE (2012) Changes in mitochondrial DNA alter expression of nuclear encoded genes associated with tumorigenesis. Exp Cell Res 318(17):2215–2225
Jandova J, Shi M, Norman KG, Stricklin GP, Sligh JE (2012) Somatic alterations in mitochondrial DNA produce changes in cell growth and metabolism supporting a tumorigenic phenotype. Biochim Biophys Acta 1822(2):293–300
Kauppila JH, Stewart JB (2015) Mitochondrial DNA: radically free of free-radical driven mutations. Biochim Biophys Acta 1847(11):1354–1361
Kurose T, Hashimoto M, Ozawa J, Kawamata S (2015) Analysis of gene expression in experimental pressure ulcers in the rat with special reference to inflammatory cytokines. PLoS One 10(7):e0132622
Menke N, Diegelmann RF (2006) Biochemical pathways of wound healing: implications for development of disease-specific diagnostics. Adv Clin Chem 41:167–187
Menke NB, Ward KR, Witten TM, Bonchev DG, Diegelmann RF (2007) Impaired wound healing. Clin Dermatol 25(1):19–25
Mookerjee SA, Goncalves RL, Gerencser AA, Nicholls DG, Brand MD (2015) The contributions of respiration and glycolysis to extracellular acid production. Biochim Biophys Acta 1847(2):171–181
Moreno-Loshuertos R, Acin-Perez R, Fernandez-Silva P, Movilla N, Perez-Martos A, de Cordoba SR, Gallardo ME, Enriquez JA (2006) Differences in reactive oxygen species production explain the phenotypes associated with common mouse mitochondrial DNA variants. Nat Genet 38(11):1261–1268
Nassiri S, Zakeri I, Weingarten MS, Spiller KL (2015) Relative expression of proinflammatory and antiinflammatory genes reveals differences between healing and nonhealing human chronic diabetic foot ulcers. J Investig Dermatol 135(6):1700–1703
Park HJ, Rouabhia M, Lavertu D, Zhang Z (2015) Electrical stimulation modulates the expression of multiple wound healing genes in primary human dermal fibroblasts. Tissue Eng Part A 21(13–14):1982–1990
Pereira GG, Guterres SS, Balducci AG, Colombo P, Sonvico F (2014) Polymeric films loaded with vitamin E and aloe vera for topical application in the treatment of burn wounds. Biomed Res Int 2014:641590
Ríos JL, Francini F, Schinella GR (2015) Natural products for the treatment of type 2 diabetes mellitus. Planta Med 81(12–13):975–994
Roy S, Khanna S, Nallu K, Hunt TK, Sen CK (2006) Dermal wound healing is subject to redox control. Mol Ther 13(1):211–220
Schafer M, Werner S (2008) Oxidative stress in normal and impaired wound repair. Pharmacol Res 58(2):165–171
Sen CK, Roy S (2008) Redox signals in wound healing. Biochim Biophys Acta 1780(11):1348–1361
Shukla A, Rasik AM, Patnaik GK (1997) Depletion of reduced glutathione, ascorbic acid, vitamin E and antioxidant defence enzymes in a healing cutaneous wound. Free Radic Res 26(2):93–101
Singer AJ, Clark RA (1999) Cutaneous wound healing. N Engl J Med 341(10):738–746
Szmydynger-Chodobska J, Strazielle N, Zink BJ, Ghersi-Egea JF, Chodobski A (2009) The role of the choroid plexus in neutrophil invasion after traumatic brain injury. J Cereb Blood Flow Metab 29(9):1503–1516
Trounce I, Wallace DC (1996) Production of transmitochondrial mouse cell lines by cybrid rescue of rhodamine-6G pre-treated L-cells. Somat Cell Mol Genet 22(1):81–85
Wlaschek M, Scharffetter-Kochanek K (2005) Oxidative stress in chronic venous leg ulcers. Wound Repair Regen 13(5):452–461
Yaman I, Derici H, Kara C, Kamer E, Diniz G, Ortac R, Sayin O (2013) Effects of resveratrol on incisional wound healing in rats. Surg Today 43(12):1433–1438
Yurdagul A, Kleinedler JJ, McInnis MC, Khandelwal AR, Spence AL, Orr AW, Dugas TR (2014) Resveratrol promotes endothelial cell wound healing under laminar shear stress through an estrogen receptor-alpha-dependent pathway. Am J Physiol Heart Circ Physiol 306(6):H797–H806
Acknowledgments
Studies presented in this manuscript were fully supported by the Dermatology Foundation research grant to Jana Jandova.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Janda, J., Nfonsam, V., Calienes, F. et al. Modulation of ROS levels in fibroblasts by altering mitochondria regulates the process of wound healing. Arch Dermatol Res 308, 239–248 (2016). https://doi.org/10.1007/s00403-016-1628-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-016-1628-9