Abstract
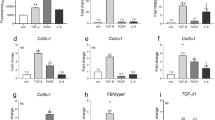
Scarring, tightly associated with fibrosis, is a significant symptomatic clinical problem. Interleukin 10 (IL-10) has been identified as a candidate scar-improving therapy based on preclinical studies. However, the molecular mechanism of IL-10 in scar improvement is still uncertain. In this study, human dermal fibroblasts stimulated with TGF-β1 were treated with IL-10 to analyze the mRNA and some of proteins’ expression levels of type I collagen (Col1), type III collagen (Col3), alpha-smooth muscle actin (α-SMA), matrix metalloproteinase-1 (MMP1), MMP2, MMP8 and tissue inhibitor of metalloproteinase 1 (TIMP1), TIMP2 by real-time PCR and Western blot, to observe α-SMA-positive fibroblasts by immunocytochemistry. The contracture and improvement of fibroblast-populated collagen lattice (FPCL) and a murine model of wound healing were used to evaluate the scar-improving effects by histological staining. The results showed that IL-10 can significantly down-regulate the mRNA and protein expression levels of Col1, Col3, α-SMA, and up-regulate the mRNA expression levels of MMP1 and MMP8, and decrease α-SMA-positive fibroblasts. FPCL analysis showed that the IL-10 (20 ng/ml) can significantly inhibit the contracture, improve the architecture of FPCL. Wounds injected with IL-10 demonstrated that the appearance of scar was improved, the wound margin of scarring was narrow, and the deposition of collagens (Col1 and Col3) in regenerated tissue was relieved. These results provide direct evidences that IL-10 has the inhibitory effects on the excessive deposition of extracellular matrix components and fibroblast-to-myofibroblast transition, and show that IL-10 has the potential therapy in prevention and reduction of skin scarring.









Similar content being viewed by others
References
Arai T, Abe K, Matsuoka H, Yoshida M, Mori M, Goya S, Kida H, Nishino K, Osaki T, Tachibana I, Kaneda Y, Hayashi S (2000) Introduction of the interleukin-10 gene into mice inhibited bleomycin-induced lung injury in vivo. Am J Physiol Lung Cell Mol Physiol 278:L914–L922
Bock O, Schmid-Ott G, Malewski P, Mrowietz U (2006) Quality of life of patients with keloid and hypertrophic scarring. Arch Dermatol Res 297:433–438
Bogdan C, Vodovotz Y, Nathan C (1991) Macrophage deactivation by interleukin 10. J Exp Med 174:1549–1555
Campaner AB, Ferreira LM, Gragnani A, Bruder JM, Cusick JL, Morgan JR (2006) Upregulation of TGF-β1 expression may be necessary but is not sufficient for excessive scarring. J Invest Dermatol 126:1168–1176
Dahlmann-Noor AH, Martin-Martin B, Eastwood M, Khaw PT, Bailly M (2007) Dynamic protrusive cell behaviour generates force and drives early matrix contraction by fibroblasts. Exp Cell Res 313:4158–4169
Desmoulière A, Geinoz A, Gabbiani F, Gabbiani G (1993) Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol 122:103–111
Diao JS, Xia WS, Yi CG, Wang YM, Li B, Xia W, Liu B, Guo SZ, Sun XD (2011) Trichostatin A inhibits collagen synthesis and induces apoptosis in keloid fibroblasts. Arch Dermatol Res 303:573–580
Eckes B, Zigrino P, Kessler D, Holtkötter O, Shephard P, Mauch C, Krieg T (2000) Fibroblast-matrix interactions in wound healing and fibrosis. Matrix Biol 19:325–332
Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore KW, O’Garra A (1991) IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol 146:3444–3451
Gabbiani G (2003) The myofibroblast in wound healing and fibrocontractive diseases. J Pathol 200:500–503
Hecker L, Jagirdar R, Jin T, Thannickal VJ (2011) Reversible differentiation of myofibroblasts by MyoD. Exp Cell Res 317:1914–1921
Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, Pennathur S, Martinez FJ, Thannickal VJ (2009) NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med 15:1077–1081
Hinz B (2007) Formation and function of the myofibroblast during tissue repair. J Investig Dermatol 127:526–537
Hinz B (2010) The myofibroblast: paradigm for a mechanically active cell. J Biomech 43:146–155
Hsu YC, Chen MJ, Yu YM, Ko SY, Chang CC (2010) Suppression of TGF-β1/SMAD pathway and extracellular matrix production in primary keloid fibroblasts by curcuminoids: its potential therapeutic use in the chemoprevention of keloid. Arch Dermatol Res 302:717–724
Kähäri VM, Saarialho-Kere U (1997) Matrix metalloproteinases in skin. Exp Dermatol 6:199–213
Kondo S (2000) The roles of cytokines in photoaging. J Dermatol Sci 23(S1):S30–S36
Leask A, Abraham DJ (2004) TGF-β signaling and the fibrotic response. FASEB J 18:816–827
LeRoy EC, Trojanowska MI, Smith EA (1990) Cytokines and human fibrosis. Eur Cytokine Netw 1:215–219
Liechty KW, Kim HB, Adzick NS, Crombleholme TM (2000) Fetal wound repair results in scar formation in interleukin-10-deficient mice in a syngeneic murine model of scarless fetal wound repair. J Pediatr Surg 35:866–872
Massague J (1998) TGF-beta signal transduction. Annu Rev Biochem 67:753–791
McCartney-Francis NL, Frazier-Jessen M, Wahl SM (1998) TGF-beta: a balancing act. Int Rev Immunol 16:553–580
Mrowietz U, Seifert O (2009) Keloid scarring: new treatments ahead. Actas Dermosifiliogr 100(Suppl 2):75–83
Nakagome K, Dohi M, Okunishi K, Tanaka R, Miyazaki J, Yamamoto K (2006) In vivo IL-10 gene delivery attenuates bleomycin induced pulmonary fibrosis by inhibiting the production and activation of TGF-β in the lung. Thorax 61:886–894
Niessen FB, Spauwen PH, Schalkwijk J, Kon M (1999) On the nature of hypertrophic scars and keloids: a review. Plast Reconstr Surg 104:1435–1458
Occleston NL, O’Kane S, Goldspink N, Ferguson MWJ (2008) New therapeutics for the prevention and reduction of scarring. Drug Discov Today 13:973–981
O’Kane S, Ferguson MW (1997) Transforming growth factor betas and wound healing. Int J Biochem Cell Biol 29:63–78
Ong CT, Khoo YT, Mukhopadhyay A, Do DV, Lim IJ, Aalami O, Phan TT (2007) mTOR as a potential therapeutic target for treatment of keloids and excessive scars. Exp Dermatol 16:394–404
Overall CM, Wrana JL, Sodek J (1989) Independent regulation of collagenase, 72-kDa progelatinase, and metalloendoproteinase inhibitor expression in human fibroblasts by transforming growth factor-beta. J Biol Chem 264:1860–1869
Park CH, Lee MJ, Ahn J, Kim S, Kim HH, Kim KH, Eun HC, Chung JH (2004) Heat shock-induced matrix metalloproteinase (MMP)-1 and MMP-3 are mediated through ERK and JNK activation and via an autocrine interleukin-6 loop. J Investig Dermatol 123:1012–1019
Peranteau WH, Zhang L, Muvarak N, Badillo AT, Radu A, Zoltick PW, Liechty KW (2008) IL-10 overexpression decrease inflammatory mediators and promotes regenerative healing in an adult model of scar formation. J Investig Dermatol 128:1852–1860
Reitamo S, Remitz A, Tamai K, Uitto J (1994) Interleukin-10 modulates type I collagen and matrix metalloprotease gene expression in cultured human skin fibroblasts. J Clin Investig 94:2489–2492
Saito M, Yamazaki M, Maeda T, Matsumura H, Setoguchi Y, Tsuboi R (2012) Pirfenidone suppresses keloid fibroblast-embedded collagen gel contraction. Arch Dermatol Res 304:217–222
Schottelius AJ, Mayo MW, Sartor RB, Baldwin AS Jr (1999) Interleukin-10 signaling blocks inhibitor of kappaB kinase activity and nuclear factor kappaB DNA binding. J Biol Chem 274:31868–31874
Seifert O, Mrowietz U (2009) Keloid scarring: bench and bedside. Arch Dermatol Res 301:259–272
Shi JH, Hu DH, Zhang ZF, Bai XZ, Wang HT, Zhu XX, Su YJ, Tang CW (2012) Reduced expression of microtubule-associated protein 1 light chain 3 in hypertrophic scars. Arch Dermatol Res 304:209–215
Sidgwick GP, Bayat A (2012) Extracellular matrix molecules implicated in hypertrophic and keloid scarring. J Eur Acad Dermatol Venereol 26:141–152
Signer AJ, Clark RA (1999) Cutaneous wound healing. N Engl J Med 341:738–746
Sternlicht MD, Werb Z (2001) How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 17:463–516
Tang B, Zhu B, Liang Y, Bi L, Hu Z, Chen B, Zhang K, Zhu J (2011) Asiaticoside suppresses collagen expression and TGF-β/Smad signaling through inducing Smad7 and inhibiting TGF-βRI and TGF-βRII in keloid fibroblasts. Arch Dermatol Res 303:563–572
Thannickal VJ, Lee DY, White ES, Cui Z, Larios JM, Chacon R, Horowitz JC, Day RM, Thomas PE (2003) Myofibroblast differentiation by transforming growth factor-beta 1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J Biol Chem 278:12384–12389
van der Veer WM, Bloemen MC, Ulrich MM, Molema G, van Zuijlen PP, Middelkoop E, Niessen FB (2009) Potential cellular and molecular causes of hypertrophic scar. Burns 35:15–29
Varga J, Jimenez SA (1986) Stimulation of normal human fibroblast collagen production and processing by transforming growth factor-beta. Biochem Biophys Res Commun 138:974–980
Vi L, de Lasa C, DiGuglilmo GM, Dagnino L (2011) Integrin-linked kinase is required for TGF-β1 induction of dermal myofibroblast differentiation. J Investig Dermatol 131:586–593
Wang P, Wu P, Siegel MI, Egan RW, Billah MM (1995) Interleukin (IL)-10 inhibits nuclear factor kappa B (NF kappa B) activation in human monocytes. IL-10 and IL-4 suppress cytokine synthesis by different mechanisms. J Biol Chem 270:9558–9563
Weiss E, Mamelak AJ, La Morgia S, Wang B, Feliciani C, Tulli A, Sander DN (2004) The role of interleukin 10 in the pathogenesis and potential treatment of skin diseases. J Am Acad Dermatol 50:657–675
Wolfram D, Tzankov A, Pülzl P, Piza-Katzer H (2009) Hypertrophic scars and keloids-a review of their pathophysiology, risk factors, and therapeutic management. Dermatol Surg 35:171–181
Yamamoto T, Eckes B, Krieg T (2001) Effect of interleukin-10 on the gene expression of type I collagen, fibronectin, and decorin in human skin fibroblasts: differential regulation by transforming growth factor-beta and monocyte chemoattractant protein-1. Biochem Biophys Res Commun 281:200–205
Yuan W, Varga J (2001) Transforming growth factor-beta repression of matrix metalloproteinase-1 in dermal fibroblasts involves Smad3. J Biol Chem 276:38502–38510
Zhang ZF, Zhang YG, Hu DH, Shi JH, Liu JQ, Zhao ZT et al (2011) Smad interacting protein 1 as a regulator of skin fibrosis in pathological scars. Burns 37:665–672
Acknowledgments
We thank Dr. Yi-Ling Zhao for histology technical assistance, Dr. Ying-Jun Su for revision the paper prior to submission, Dr. Na Li for skilled animal technical assistance, and Dr. Yao-Jun Wang for processing the figures. The work was supported by the Grant-in-Aid for scientific research from Xijing hospital assist in 2010 (XJZT10D04), and the National Natural Science Foundation of China (Grant Number 81272098).
Author information
Authors and Affiliations
Corresponding author
Additional information
J.-H. Shi, H. Guan and S. Shi contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shi, JH., Guan, H., Shi, S. et al. Protection against TGF-β1-induced fibrosis effects of IL-10 on dermal fibroblasts and its potential therapeutics for the reduction of skin scarring. Arch Dermatol Res 305, 341–352 (2013). https://doi.org/10.1007/s00403-013-1314-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-013-1314-0