Abstract
Introduction
The availability of autogenous tendons (middle part of patellar tendon, semitendinosus/gracilis, or quadriceps tendon) for cruciate ligament reconstructions is restricted and related to withdrawal morbidity. Allografts and synthetic ligament materials often show problems regarding long-term stability and immunological reactions. Therefore, the aim of this study was to develop and characterize a new scaffold based on acellular allografts seeded with autologous cells for tissue engineering of the anterior cruciate ligament (ACL).
Materials and methods
Semitendinosus tendons of New Zealand White (NZW) rabbits were harvested and acellularized using the detergent sodium dodecyle sulfate (SDS) as the main ingredient. After that, cultured (37°C, 5% CO2, medium) dermal fibroblasts were injected into the tendons. These constructs were further cultivated for 4, 7, or 14 days under the same culture conditions. Native, acellular, and seeded tendons underwent biomechanical testing (ultimate load to failure [N], stiffness [N/mm], and elongation [%], each n = 9] and histological hematoxylin-eosin (H.E.) staining. Detailed immunohistochemical (collagen I, III, IV, VI, pro-collagen I, versican, and vimentin) analyses were conducted to detect changes in the composition and structure of the extracellular matrix (ECM) after acellularization.
Results
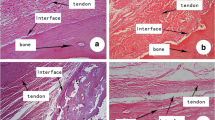
Histologically, a cell-free, crimped slack tendon structure after acellularization and a good integration of the cells after injection (4, 7, and 14 days) were seen. Metabolic activity of the seeded cells was demonstrated by positive immunohistochemical staining for pro-collagen I, which was negative in nonseeded constructs. Major differences in staining patterns of the various other ECM components were not observed. Biomechanically, the maximum load to failure of these tendons was comparable to native tendons (P = 0.429; native 134.5 ± 12.9 N; acellular 118.5 ± 7.3 N; seeded 132.3 ± 5.6 N). Stiffness and elongation were comparable between native and acellular tendons, but differed significantly after seeding (P < 0.001).
Conclusion
The described method is suitable to make tendons completely cell free without changing their major biomechanical properties. Preservation of the ECM and of the collagen fiber structure by this method should give an ideal environment for autologous cell integration and metabolic activity in contrast to other approaches for tissue acellularization. The cell disruption and extraction of cell detritus should minimize adverse immunogenic reactions.




Similar content being viewed by others
References
Allaire E, Guettier C, Bruneval P, Plissonnier D, Michel JB (1994) Cell-free arterial grafts: morphologic characteristics of aortic isografts, allografts, and xenografts in rats. J Vasc Surg 19:446–456
Altman GH, Horan RL, Lu HH, Moreau J, Martin I, Richmond JC, Kaplan DL (2002) Silk matrix for tissue engineered anterior cruciate ligaments. Biomaterials 23:4131–41
Bellincampi LD, Closkey RF, Prasad R, Zawadsky JP, Dunn MG (1998) Viability of fibroblast-seeded ligament analogs after autogenous implantation. J Orthop Res 16:414–420
Beynnon BD, Johnson RJ, Abate JA, Fleming BC, Nichols CE (2005) Treatment of anterior cruciate ligament injuries, part 2. Am J Sports Med 33:1751–67
Beynnon BD, Johnson RJ, Abate JA, Fleming BC, Nichols CE (2005) Treatment of anterior cruciate ligament injuries, part I. Am J Sports Med 33:1579–602
Bhrany AD, Beckstead BL, Lang TC, Farwell DG, Giachelli CM, Ratner BD (2006) Development of an esophagus acellular matrix tissue scaffold. Tissue Eng 12:319–30
Cartmell JS, Dunn MG (2004) Development of cell-seeded patellar tendon allografts for anterior cruciate ligament reconstruction. Tissue Eng 10:1065–75
Cartmell JS, Dunn MG (2000) Effect of chemical treatments on tendon cellularity and mechanical properties. J Biomed Mater Res 49:134–140
Cole DW, Ginn TA, Chen GJ, Smith BP, Curl WW, Martin DF, Poehling GG (2005) Cost comparison of anterior cruciate ligament reconstruction: autograft versus allograft. Arthroscopy 21:786–90
Durselen L, Claes L, Ignatius A, Rubenacker S (1996) Comparative animal study of three ligament prostheses for the replacement of the anterior cruciate and medial collateral ligament. Biomaterials 17:977–82
Gentleman E, Livesay GA, Dee KC, Nauman EA (2006) Development of Ligament-Like Structural Organization and Properties in Cell-Seeded Collagen Scaffolds in vitro. Ann Biomed Eng:1–11
Gilbert TW, Sellaro TL, Badylak SF (2006) Decellularization of tissues and organs. Biomaterials 27:3675–83
Goldstein S, Clarke DR, Walsh SP, Black KS, O’Brien MF (2000) Transpecies heart valve transplant: advanced studies of a bioengineered xeno-autograft. Ann Thorac Surg 70:1962–9
Gorschewsky O, Klakow A, Riechert K, Pitzl M, Becker R (2005) Clinical comparison of the Tutoplast allograft and autologous patellar tendon (bone-patellar tendon-bone) for the reconstruction of the anterior cruciate ligament: 2- and 6-year results. Am J Sports Med 33:1202–9
Harrison RD, Gratzer PF (2005) Effect of extraction protocols and epidermal growth factor on the cellular repopulation of decellularized anterior cruciate ligament allografts. J Biomed Mater Res A
Hefti FL, Kress A, Fasel J, Morscher EW (1991) Healing of the transected anterior cruciate ligament in the rabbit. J Bone Joint Surg Am 73:373–83
Jackson DW, Grood ES, Arnoczky SP, Butler DL, Simon TM (1987) Freeze dried anterior cruciate ligament allografts. Preliminary studies in a goat model. Am J Sports Med 15:295–303
Kadler KE, Holmes DF, Trotter JA, Chapman JA (1996) Collagen fibril formation. Biochem J 316(Pt 1):1–11
Milthorpe BK (1994) Xenografts for tendon and ligament repair. Biomaterials 15:745–52
Milz S, Benjamin M, Putz R (2005) Molecular parameters indicating adaptation to mechanical stress in fibrous connective tissue. Adv Anat Embryol Cell Biol 178:1–71
Mirsadraee S, Wilcox HE, Korossis SA, Kearney JN, Watterson KG, Fisher J, Ingham E (2006) Development and characterization of an acellular human pericardial matrix for tissue engineering. Tissue Eng 12:763–73
Muren O, Dahlstedt L, Brosjo E, Dahlborn M, Dalen N (2005) Gross osteolytic tibia tunnel widening with the use of Gore-Tex anterior cruciate ligament prosthesis: a radiological, arthrometric and clinical evaluation of 17 patients 13–15 years after surgery. Acta Orthop 76:270–4
Olesen JL, Langberg H, Heinemeier KM, Flyvbjerg A, Kjaer M (2006) Determination of markers for collagen type I turnover in peritendinous human tissue by microdialysis: effect of catheter types and insertion trauma. Scand J Rheumatol 35:312–317
Petrigliano FA, McAllister DR, Wu BM (2006) Tissue engineering for anterior cruciate ligament reconstruction: a review of current strategies. Arthroscopy 22:441–51
Poehling GG, Curl WW, Lee CA, Ginn TA, Rushing JT, Naughton MJ, Holden MB, Martin DF, Smith BP (2005) Analysis of outcomes of anterior cruciate ligament repair with 5-year follow-up: allograft versus autograft. Arthroscopy 21:774–85
Roolker W, Patt TW, van Dijk CN, Vegter M, Marti RK (2000) The Gore-Tex prosthetic ligament as a salvage procedure in deficient knees. Knee Surg Sports Traumatol Arthrosc 8:20–5
Tischer T, Milz S, Maier M, Schieker M, Benjamin M (2002) An immunohistochemical study of the rabbit suprapatella, a sesamoid fibrocartilage in the quadriceps tendon containing aggrecan. J Histochem Cytochem 50:955–960
Van Eijk F, Saris DB, Riesle J, Willems WJ, Van Blitterswijk CA, Verbout AJ, Dhert WJ (2004) Tissue engineering of ligaments: a comparison of bone marrow stromal cells, anterior cruciate ligament, and skin fibroblasts as cell source. Tissue Eng 10:893–903
Walter RJ, Matsuda T, Reyes HM, Walter JM, Hanumadass M (1998) Characterization of acellular dermal matrices (ADMs) prepared by two different methods. Burns 24:104–13
Woods T, Gratzer PF (2005) Effectiveness of three extraction techniques in the development of a decellularized bone-anterior cruciate ligament-bone graft. Biomaterials 26:7339–49
Zimmerman MC, Contiliano JH, Parsons JR, Prewett A, Billotti J (1994) The biomechanics and histopathology of chemically processed patellar tendon allografts for anterior cruciate ligament replacement. Am J Sports Med 22:378–86
Acknowledgments
Funds from Commission for Clinical Research (KKF) of the Technical University Munich, Germany, provided support for the research presented in this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
T. Tischer and S. Vogt contributed equally to this work.
Rights and permissions
About this article
Cite this article
Tischer, T., Vogt, S., Aryee, S. et al. Tissue engineering of the anterior cruciate ligament: a new method using acellularized tendon allografts and autologous fibroblasts. Arch Orthop Trauma Surg 127, 735–741 (2007). https://doi.org/10.1007/s00402-007-0320-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-007-0320-0