Abstract
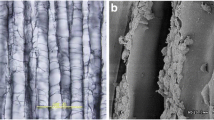
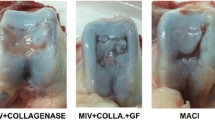
Introduction: Matrix-associated transplantation of cartilage constructs is an appealing method in cartilage repair. Three different matrices seeded with allogenic chondrocytes were compared in an osteochondral defect model in the rabbit. An investigation was conducted to identify the best matrix for cell-based treatment of osteochondral defects in the rabbit knee joint. Materials and methods: Osteochondral defects (diameter 3 mm) were created in the trochlea and the femoral condyles of 33 New Zealand White rabbits, which were then treated with bioartificial cartilage constructs. The cartilage constructs were created in vitro using three different resorbable carrier materials (two fleece matrices: one of PLLA, and one composite of polydioxanon/polyglactin, as well as one consisting of lyophilized dura) cultured with isolated allogenic chondrocytes. The defects were evaluated macroscopically, by histological and immunhistological techniques, and by scanning electron microscopy after 6 weeks, 6 months, and 12 months. The chondrocyte-seeded constructs were compared to defects treated with carrier material alone as well as to untreated control defects. Results: There was a significant improvement in defect repair quality in the transport materials, which were cultured with chondrocytes prior to implantation (P<0.0005). No significant differences were observed between the three carrier matrices, and no significant differences were seen between the unseeded matrices and the untreated control defects. Conclusion: There is no difference in the outcome between the three tested matrices in the treatment of osteochondral defects in the rabbit knee. The results of this in vitro experiment are promising and with refinement may lead to useful clinical therapies.



Similar content being viewed by others
References
Ball ST, Goomer RS, Ostrander RV, Tontz WL Jr, Williams SK, Amiel D (2004) Preincubation of tissue engineered constructs enhances donor cell retention. Clin Orthop 420:276–285
Breinan HA, Martin SD, Hsu HP, Spector M (2000) Healing of canine articular cartilage defects treated with microfracture, a type-II collagen matrix, or cultured autologous chondrocytes. J Orthop Res 18:781–789
Breinan HA, Minas T, Hsu HP, Nehrer S, Sledge CB, Spector M (1997) Effect of cultured autologous chondrocytes on repair of chondral defects in a canine model. J Bone Joint Surg [Am] 79:1439–1451
Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L (1994) Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 331:889–895
Brittberg M, Nilsson A, Lindahl A, Ohlsson C, Peterson L (1996) Rabbit articular cartilage defects treated with autologous cultured chondrocytes. Clin Orthop 326:270–283
Bruns J, Kersten P, Lierse W, Silbermann M (1992) Autologous rib perichondral grafts in experimentally induced osteochondral lesions in the sheep knee joint: morphological results. Virchows Arch 421:1–8
Butnariu-Ephrat M, Robinson D, Mendes DG, Halperin N, Nevo Z (1996) Resurfacing of goat articular cartilage by chondrocytes derived from bone marrow. Clin Orthop 330:234–243
Cancedda FD, Gentili C, Manduca P, Cancedda R (1992) Hypertrophic chondrocytes undergo further differentiation in culture. J Cell Biol 117:427–435
Chu CR, Coutts RD, Yoshioka M, Harwood FL, Monosov AZ, Amiel D (1995) Articular cartilage repair using allogeneic perichondrocyte-seeded biodegradable porous polylactic acid (PLA): a tissue-engineering study. J Biomed Mater Res 29:1147–1154
Daniels K, Solursh M (1991) Modulation of chondrogenesis by the cytoskeleton and extracellular matrix. J Cell Sci 100:249–254
Domm C, Fay J, Schünke M, Kurz B (2000) Die Redifferenzierung von dedifferenzierten Gelenkknorpelzellen in Alginatkultur. Einfluss von intermittierendem hydrostatischen Druck und niedrigem Sauerstoffpartialdruck. Orthopäde 29:91–99
Fragonas E, Valente M, Pozzi-Mucelli M, Toffanin R, Rizzo R, Silvestri F, Vittur F (2000) Articular cartilage repair in rabbits by using suspensions of allogenic chondrocytes in alginate. Biomaterials 21:795–801
Freed LE, Grande DA, Lingbin Z, Emmanual J, Marquis JC, Langer R (1994) Joint resurfacing using allograft chondrocytes and synthetic biodegradable polymer scaffolds. J Biomed Mater Res 28:891–899
Freed LE, Marquis JC, Nohria JC, Emmanual J, Mikos AG, Langer R (1993) Neocartilage formation in vitro and in vivo using cells cultured on synthetic biodegradable polymers. J Biomed Mater Res 27:11–23
Frenkel SR, Toolan BC, Menche D, Pitman MI, Pachence JM (1997) Chondrocyte transplantation using a collagen bilayer matrix for cartilage repair. J Bone Joint Surg [Br] 79:831–836
Gao J, Dennis JE, Solchaga LA, Goldberg VM, Caplan AI (2002) Repair of osteochondral defect with tissue-engineered two-phase composite material of injectable calcium phosphate and hyaluronan sponge. Tissue Eng 8:827–837
Grande DA, Pitman MI, Peterson L, Menche D, Klein M (1989) The repair of experimentally produced defects in rabbit articular cartilage by autologous chondrocyte transplantation. J Orthop Res 7:208–218
Helbing G (1982) Transplantation isolierter Chondrozyten in Gelenkknorpel-Defekte. Fortschr Med 100:83–87
Hendrickson DA, Nixon AJ, Grande DA, Todhunter RJ, Minor RM, Erb H, Lust G (1994) Chondrocyte-fibrin matrix transplants for resurfacing extensive articular cartilage defects. J Orthop Res 12:485–497
Hirschmann F, Verhoeyen E, Wirth D, Bauwens S, Hauser H, Rudert M (2002) Vital marking of articular chondrocytes by retroviral infection using green fluorescence protein. Osteoarthritis Cartilage 10:109–118
Holmdahl R, Rubin K, Klareskog L, Larsson E, Wigzell H (1986) Characterization of the antibody response in mice with type II collagen-induced arthritis, using monoclonal anti-type II collagen antibodies. Arthritis Rheum 29:400–410
Hunter W (1743) On the structure and diseases of articulating cartilages. Philos Trans R Soc 42:514–521
Hunziker EB, Driesang IM (2003) Functional barrier principle for growth-factor-based articular cartilage repair. Osteoarthritis Cartilage 11:320–327
Im GI, Kim DY, Shin JH, Hyun CW, Cho WH (2001) Repair of cartilage defect in the rabbit with cultured mesenchymal stem cells from bone marrow. J Bone Joint Surg Br 83:289–294
Katsube K, Ochi M, Uchio Y, Maniwa S, Matsusaki M, Tobita M, Iwasa J (2000) Repair of articular cartilage defects with cultured chondrocytes in Atelocollagen gel. Comparison with cultured chondrocytes in suspension. Arch Orthop Trauma Surg 120:121–127
Kawamura S, Wakitani S, Kimura T, Maeda A, Caplan AI, Shino K, Ochi T (1998) Articular cartilage repair. Rabbit experiments with a collagen gel-biomatrix and chondrocytes cultured in it. Acta Orthop Scand 69:56–62
Kölliker A (1867) Handbuch der Gewebelehre des Menschen. Engelmann Leipzig 5:64–69
Lee CR, Grodzinsky AJ, Hsu HP, Spector M (2003) Effects of a cultured autologous chondrocyte-seeded type II collagen scaffold on the healing of a chondral defect in a canine model. J Orthop Res 21:272–281
Melamed E, Robinson D, Halperin N, Nevo Z (2004) Restoration of arthritic cartilage defects using autologous chondrocytes transplantation is superior to cartilage-paste graft in rabbits. J Knee Surg 17:6–12
Nehrer S, Breinan HA, Ramappa A, Hsu HP, Minas T, Shortkroff S, Sledge CB, Yannas IV, Spector M (1998) Chondrocyte-seeded collagen matrices implanted in a chondral defect in a canine model. Biomaterials 19:2313–2328
Niederauer GG, Slivka MA, Leatherbury NC, Korvick DL, Harroff HH Jr, Ehler WC, Dunn CJ, Kieswetter K (2000) Evaluation of multiphase implants for repair of focal osteochondral defects in goats. Biomaterials 21:2561–2574
Noguchi T, Oka M, Fujino M, Neo M, Yamamuro T (1994) Repair of osteochondral defects with grafts of cultured chondrocytes. Comparison of allografts and isografts. Clin Orthop 302:251–258
O’Driscoll SW (1999) Articular cartilage regeneration using periosteum. Clin Orthop 367(Suppl):S186–S203
O’Driscoll SW, Keeley FW, Salter RB (1988) Durability of regenerated articular cartilage produced by free autogenous periosteal grafts in major full-thickness defects in joint surfaces under the influence of continuous passive motion A follow-up report at one year. J Bone Joint Surg [Am] 70:595–606
Paget J (1853) Healing of injuries in various tissues. Lect Surg Pathol 1:262–291
Perka C, Schultz O, Sittinger M, Zippel H (2000) Chondrozytentransplantation in PGLA Polydioxanon-Vliesen. Orthopäde 29:112–119
Pineda S, Pollack A, Stevenson S, Goldberg VM, Caplan AI (1992) A semiquantitative scale for histologic grading of articular cartilage repair. Acta Anat 143:335–340
Qiu YS, Shahgaldi BF, Revell WJ, Heatley FW (2003) Observations of subchondral plate advancement during osteochondral repair: a histomorphometric and mechanical study in the rabbit femoral condyle. Osteoarthritis Cartilage 11:810–820
Rahfoth B, Weisser J, Sternkopf F, Aigner T, von der M, Brauer R (1998) Transplantation of allograft chondrocytes embedded in agarose gel into cartilage defects of rabbits. Osteoarthritis Cartilage 6:50–65
Reinholz GG, Lu L, Saris DB, Yaszemski MJ, O’Driscoll SW (2004) Animal models for cartilage reconstruction. Biomaterials 25:1511–1521
Rudert M (2002) Histological evaluation of osteochondral defects: consideration of animal models with emphasis on the rabbit, experimental setup, follow-up and applied methods. Cells Tissues Organs 171:229–240
Rudert M (2003) Kultur und Transplantation von Chondrozyten auf Polymer-Vliesen und lyophylisierter Dura. Biomaterialien 4:31–37
Rudert M, Hirschmann F, Schulze M, Wirth CJ (2000) Bioartificial cartilage. Cells Tissues Organs 167:95–105
Rudert M, Hirschmann F, Wirth CJ (1999) Wachstumsverhalten von Chondrozyten auf unterschiedlichen Trägersubstanzen. Orthopäde 28:68–75
Rudert M, Wirth CJ, Schulze M, Reiss G (1998) Synthesis of articular cartilage like tissue in vitro. Arch Orthop Trauma Surg 117:141–146
Sams AE, Minor RR, Wootton JA, Mohammed H, Nixon AJ (1995) Local and remote matrix responses to chondrocyte-laden collagen scaffold implantation in extensive articular cartilage defects. Osteoarthritis Cartilage 3:61–70
Schreiber RE, Ilten-Kirby BM, Dunkelman NS, Symons KT, Rekettye LM, Willoughby J, Ratcliffe A (1999) Repair of osteochondral defects with allogeneic tissue engineered cartilage implants. Clin Orthop 367 Suppl:S382–S395
Shahgaldi BF, Amis AA, Heatley FW, McDowell J, Bentley G (1991) Repair of cartilage lesions using biological implants. A comparative histological and biomechanical study in goats. J Bone Joint Surg [Br] 73:57–64
Shapiro F, Koide S, Glimcher MJ (1993) Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Joint Surg [Am] 75:532–553
Solchaga LA, Gao J, Dennis JE, Awadallah A, Lundberg M, Caplan AI, Goldberg VM (2002) Treatment of osteochondral defects with autologous bone marrow in a hyaluronan-based delivery vehicle. Tissue Eng 8:333–347
Stockwell RA (1979) Chondrogenesis and chondrocyte differentiation. In: Harrison RJ, McMinn RMH (eds) Biology of cartilage cells. Cambridge University Press, Cambridge, 179–212
Vacanti CA, Kim WS, Schloo B, Upton J, Vacanti JP (1994) Joint resurfacing with cartilage grown in situ from cell-polymer structures. Am J Sports Med 22:485–488
van Susante JL, Buma P, Schuman L, Homminga GN, van den Berg WB, Veth RP (1999) Resurfacing potential of heterologous chondrocytes suspended in fibrin glue in large full-thickness defects of femoral articular cartilage: an experimental study in the goat. Biomaterials 20:1167–1175
von der Mark K (1986) Differentiation, modulation and dedifferentiation of chondrocytes. Rheumatology 10:272–315
Wakitani S, Goto T, Pineda SJ, Young RG, Mansour JM, Caplan AI, Goldberg VM (1994) Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg [Am] 76:579–592
Wakitani S, Kimura T, Hirooka A, Ochi T, Yoneda M, Yasui N, Owaki H, Ono K (1989) Repair of rabbit articular surfaces with allograft chondrocytes embedded in collagen gel. J Bone Joint Surg [Br] 71:74–80
Weisser J, Rahfoth B, Timmermann A, Aigner T, Bräuer R, von der Mark K (2001) Role of growth factors in rabbit articular cartilage repair by chondrocytes in agarose. Osteoarthritis Cartilage 9(Suppl A):48–54
Werkmeister JA, Ramshaw JA (1989) Monoclonal antibodies to collagens for immunofluorescent examination of human skin. Acta Derm Venereol 69:399–402
Wirth CJ, Rudert M (1996) Techniques of cartilage growth enhancement: a review of the literature. Arthroscopy 12:300–308
Acknowledgments
This study was funded in part by grants from the Deutsche Forschungsgemeinschaft (DFG-Wi 494/14–1) and by the Deutsche Arthrose-Hilfe. We thank Prof. Dr. M. Schünke, Institute of Anatomy, University of Kiel, Germany for his invaluable help in the preparation of the collagen immunohistochemistry and Dr. H. Herrmann, Institute of Biometry, Medical School Hannover, Germany for his help in the statistical analysis. The animal experiments were approved by the ethics commission of the District Government of Lower-Saxony (No. 604-42502-96/835).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rudert, M., Wilms, U., Hoberg, M. et al. Cell-based treatment of osteochondral defects in the rabbit knee with natural and synthetic matrices: cellular seeding determines the outcome. Arch Orthop Trauma Surg 125, 598–608 (2005). https://doi.org/10.1007/s00402-005-0008-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-005-0008-2